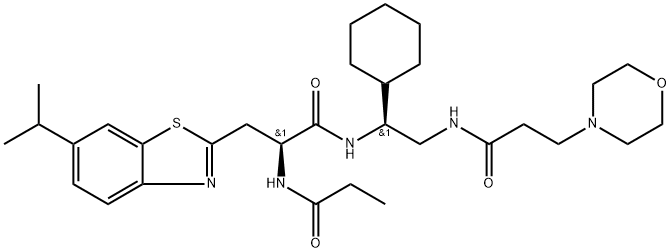
DI-591 is a potent, high-affinity and cell-permeable inhibitor of the DCN1-UBC12 interaction. DI-591 binds to DCN1 and DCN2 with Ki values of 12 nM and 10.4 nM, respectively and has no appreciable binding to DCN3, DCN4, and DCN5 proteins. DI-591 selectively inhibits neddylation of cullin 3 but has no or minimal effect on neddylation of other cullin family members[1].
DI-591 (Compound 44) binds to DCN1 and DCN2 with Ki values of 12 nM and 10.4 nM, respectively and has no appreciable binding to DCN3, DCN4, and DCN5 proteins. Hence, DI-591 displays a very-high binding affinity to recombinant human DCN1 and DCN2 proteins and >1000-fold selectivity over recombinant human DCN3-5 proteins[1].DI-591 (Compound 44; 0-10 μM; 1 hour; KYSE70 cells) binds to both cellular DCN1 and DCN2 proteins and disrupts the association of cellular DCN1 and UBC12 proteins[1].DI-591 (Compound 44; 10 μM; 24 hours; THLE2 cells) treatment robustly increases the mRNA levels of NQO1 and HO1, leading to upregulation of HO1 protein in the cells. Significantly, DI-591 has no effect on the mRNA level of NRF2[1].The selective inhibition of neddylation of cullin 3 by DI-591 leads to accumulation NRF2 protein and its transcriptional activation. Knockdown experiments indicate that DCN1, but not DCN2, plays a key role in regulation of neddylation of cullin 3 but not of other cullins. DI-591 is an excellent probe compound to investigate the role of the cullin 3 CRL (Cullin-RING E3 ubiquitin ligase) in biological processes and human diseases[1].