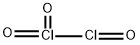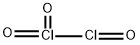Dichlorine trioxide, Cl2O3, is a chlorine oxide. It is a dark brown solid discovered in 1967 which is explosive even below 0 °C. It is formed by the low-temperature photolysis of ClO2 and is formed along with Cl2O6, Cl2 and O2. Its structure is believed to be OCl?ClO2 with possible isomers such as Cl?O?ClO2. The isomer having a structure of OCl–O–ClO would be the theoretical anhydride of chlorous acid.
Dichlorine trioxide, Cl2O3, is a chlorine oxide that is
the anhydride of chlorous acid. It is a dark brown gas
discovered in 1967 which is explosive even below 0°C.
It is formed by the low-temperature photolysis of ClO2
and is formed along with Cl2O6, Cl2 and O2. Its structure
is believed to be OCl–ClO2 with possible isomers such as
Cl–O–ClO2. It is very soluble and forms chlorous acid if
dissolved in water:
Cl2O3 (gas)+H2O→2H2ClO2 (aq)
It is less stable than chlorous acid, its more stable
product, and therefore is not used commercially as an
oxidizing agent. Its solubility in water is low, about
3.42 g/100 ml of water but it slowly reacts to form chlorous acid at room temperature. Raising the temperature causes it to decompose to form chlorine gas that remains soluble in aqueous solution.