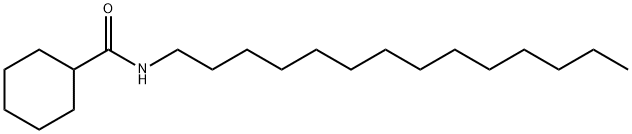
Numerous analogs of fatty acyl ethanolamides potentiate the intrinsic biological activity of endocannabinoids.
1 This potentiation is ascribed either to inhibition of AEA reuptake into neurons, or inhibition of fatty acid amide hydrolase (FAAH) within the neurons.
2 However, Ueda,
et al. has recently cloned another amidase, the acidic PEAase that promotes the hydrolysis of palmitoylethanolamide.
3 N-
Cyclohexanecarbonyltetradecylamine is an analog of N-
cyclohexanecarbonyl-
pentadecylamine, a selective inhibitor of acidic PEAase with an IC
50 value of 4.5 μM, that contains 1 less carbon in the alkyl chain.
4 The biological activity of N-
cyclohexanecarbonyltetradecylamine has not been documented.
1. Khanolkar, A.D., and Makriyannis, A. Structure-activity relationships of anandamide, an endogenous cannabinoid ligand Life Sci. 65,607-616(1999).
2. Deutsch, D.G., Glaser, S.T., Howell, J.M., et al. The cellular uptake of anandamide is coupled to its breakdown by fatty-acid amide hydrolase J. Biol. Chem. 276(10),6967-6973(2001).
3. Ueda, N., Yamanaka, K., and Yamamoto, S. Purification and characterization of an acid amidase selective for N-palmitoylethanolamine, a putative endogenous anti-inflammatory substance J. Biol. Chem. 276(38),35552-35557(2001).
4. Tsuboi, K., Hilligsmann, C., Vandevoorde, S., et al. N-cyclohexanecarbonylpentadecylamine: A selective inhibitor of the acid amidase hydrolysing N-acylethanolamines, as a tool to distinguish acid amidase from fatty acid amide hydrolase Biochem. J. 379,99-106(2004).