BIO 5192
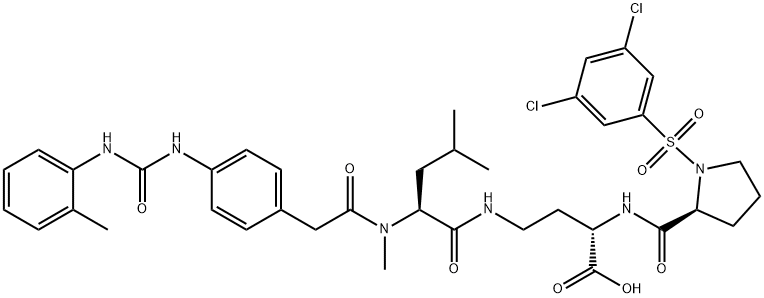
BIO 5192 性质
| 熔点 | 134 - 136°C |
|---|---|
| 密度 | 1.380±0.06 g/cm3(Predicted) |
| 储存条件 | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| 溶解度 | 二甲基亚砜(微溶) |
| 形态 | 固体 |
| 酸度系数(pKa) | 3.52±0.10(Predicted) |
| 颜色 | 白色至类白色 |
BIO 5192 用途与合成方法
|
α4β1 1.8 nM (IC 50 ) |
α9β1 138 nM (IC 50 ) |
α2β1 1053 nM (IC 50 ) |
α4β7 >500 nM (IC 50 ) |
The combination of BIO5192 (1 mg/kg; i.v.) and Plerixafor (5 mg/kg; s.c.) exert an additive effect on progenitor mobilization.
BIO5192 (30 mg/kg; s.c; bid; during days 5 through 14) delays paralysis associated with EAE (experimental autoimmune encephalomyelitis).
BIO5192 (1 mg/kg, i.v.) shows the terminal half-life is 1.1 hours. BIO5192 (3, 10, and 30 mg/kg; s.c.) shows half-lives of 1.7, 2.7, and 4.7 hours, respectively. The blood plasma curves show that the AUC for the s.c. route of administration increased about 2.5-fold from 5,460 h*ng/ml for the 3 mg/kg dose to 14,175 h*ng/ml for the 30 mg/kg.
| Animal Model: | C57BL/6J x 129Sv/J F1 mice |
| Dosage: | 1 mg/kg (with Plerixafor: 5 mg/kg) |
| Administration: | I.v. |
| Result: | Exerted an additive effect on progenitor mobilization. |
| Animal Model: | Healthy female Lewis rats weighing 150g |
| Dosage: | 30 mg/kg |
| Administration: | S.c; bid; during days 5 through 14 |
| Result: | Showed a 3-day delay in onset of disease. |
BIO 5192 价格(试剂级)
| 更新日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
|---|---|---|---|---|---|
| 2024-11-08 | HY-107589 | BIO 5192 | 327613-57-0 | 5 mg | 2200 |
| 2024-11-08 | HY-107589 | BIO 5192 | 327613-57-0 | 10 mg | 3500 |

