LSF, a chiral metabolite of pentoxifylline, acts as a potent anti-inflammatory agent. (S)-LSF is the pharmacologically inactive optical enantiomer of (R)-LSF, the biologically active isomer. When metabolized by isolated human liver cells, pentoxifylline is exclusively reduced to (S)-LSF in the cytosol, while reduction in liver microsomes is 85% stereoselective in favor of (S)-LSF formation. Thus, pentoxifylline is an inefficient prodrug for the delivery of therapeutically relevant lisofylline.
LSF, a chiral metabolite of pentoxifylline, acts as a potent anti-inflammatory agent. (S)-LSF is the pharmacologically inactive optical enantiomer of (R)-LSF, the biologically active isomer. When metabolized by isolated human liver cells, pentoxifylline is exclusively reduced to (S)-LSF in the cytosol, while reduction in liver microsomes is 85% stereoselective in favor of (S)-LSF formation. Thus, pentoxifylline is an inefficient prodrug for the delivery of therapeutically relevant lisofylline.[Cayman Chemical]
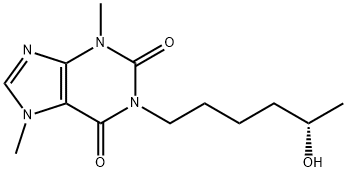
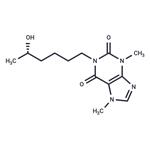
ChEBI: (S)-lisofylline is a 1-(5-hydroxyhexyl)-3,7-dimethyl-3,7-dihydro-1H-purine-2,6-dione that has (S)-configuration. It is the inactive optical enantiomer of (R)-lisofylline, an anti-inflammatory agent. It is an enantiomer of a (R)-lisofylline.
in rats subjected to hemorrhagic shock and resuscitation, lsf increased the intestinal and hepatic blood flow. treatment with lsf (15 mg/kg) ameliorated the development of mucosal damage and hyperpermeability. rats treated with lsf showed lower plasma concentrations of the intracellular hepatic enzyme, aspartate aminotransferase. lsf treatment increased concentrations of adenosine triphosphate in intestinal and hepatic tissue [1]. in nod mice, lisofylline suppressed ifn-γ production, reduced the onset of insulitis and diabetes, and inhibited diabetes after transfer of splenocytes from lisofylline-treated donors to nod.scid recipients [3].
[1] wattanasirichaigoon s, menconi m j, fink m p. lisofylline ameliorates intestinal and hepatic injury induced by hemorrhage and resuscitation in rats[j]. critical care medicine, 2000, 28(5): 1540-1549.
[2] lillibridge, j. a.,kalhorn, t.f. and slattery, j.t. metabolism of lisofylline and pentoxifylline in human liver microsomes and cytosol. drug metabolism and disposition 24(11), 1174-1179 (1996).
[3] yang z d, chen m, wu r, et al. the anti-inflammatory compound lisofylline prevents type i diabetes in non-obese diabetic mice[j]. diabetologia, 2002, 45(9): 1307-1314.
[4] chen m, yang z, wu r, et al. lisofylline, a novel antiinflammatory agent, protects pancreatic β-cells from proinflammatory cytokine damage by promoting mitochondrial metabolism[j]. endocrinology, 2002, 143(6): 2341-2348.