Standard
In the analysis work, it often demanded to apply high-quality compound with the exact content being accurate in order to be compared as the detection control or used to calibrate the instrument, the compound is known as standard sample.
Standard refers to the standard substance used for measuring the potency of biological products. When the production department submits to a higher authority for approval of the quality standard of the biological product, they should also provide the standard sample (indicated in unit (μ)) used for measuring the potency of the tested product.
According to different application, the verification products can be divided into biological standard for the testing of biological products, endocrine drugs, antibiotics and other bioassay and chemical standards including:
1 chemical standards used for calibration of the instrument;
2 chemical reference standards used for calibration of the ultraviolet or infrared spectrum of the drugs;
3 control standards used for the purity test of drugs as well as the impurity control or as the control standards for content determination of the medicine.
In the page 67 of the appendix in the People's Republic of China Pharmacopoeia 1990 1st edition, we can find hundreds kinds of standards including bornyl ester acetate, eugenol and panaxadiol as well as 39 kinds of control medicine for the purify inspection of traditional Chinese medicine, impurity control or the content determination.
International standards
This refers to standard sample, with the potency units of the existing international standard sample potency units as baseline, undergoing further calibration of its potency units on the basis of a wide range of international cooperation. Its potency is expressed in 1U. For a certain product, for the establishment of the first international standard sample, the determination of its potency units should be consulted and decided by the Committee of Experts before the announcement. The international standard is a standard reference substance for worldwide preparation of national standards, so that the international standards can achieve unity.
Reagents and standards
All reagents in the food analysis laboratories should be clearly labeled to indicate the following: 1 name; 2 the date of preparation; 3 Signature from the people in charge; 4 if necessary, we need to add content such as safety and validity and so on.
Most food analysis should use standard sample as reference for qualitative and quantitative determination. The standard sample should be classified according to the following classification methods: 1 international standard; 2 national standards; 3 internal standard; 4 other standards.
Receptacle for reference standards should be clearly affixed to the label, to indicate the following: 1 name; 2 standard document number; 3 Opening times; 4 Validity; 5 Signature.
In addition, the laboratory should keep all the necessary specific files for all the necessary reference standards.
Capacity analysis standard solution should also be strictly managed. It should be clearly posted on all the standard solution containers with labels to indicate: 1 name; 2 concentration; 3 formulation date; 4 No of formulated procedures; 5 storage conditions; 6 validity; 7 Signature from people in charge.
- Structure:
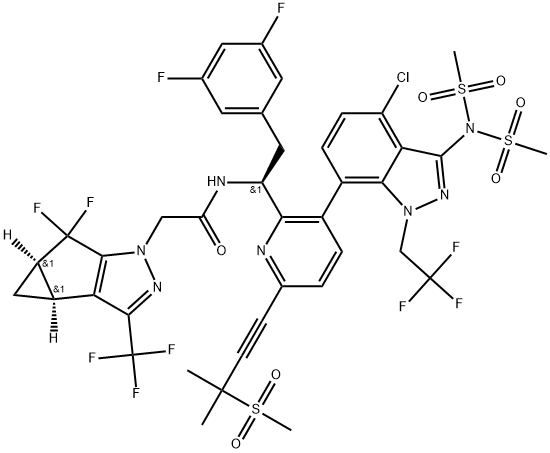
- Chemical Name:1H-Cyclopropa[3,4]cyclopenta[1,2-c]pyrazole-1-acetamide, N-[(1S)-1-[3-[3-[bis(methylsulfonyl)amino]-4-chloro-1-(2,2,2-trifluoroethyl)-1H-indazol-7-yl]-6-[3-methyl-3-(methylsulfonyl)-1-butyn-1-yl]-2-pyridinyl]-2-(3,5-difluorophenyl)ethyl]-5,5-difluoro-3b,4,4a,5-tetrahydro-3-(trifluoromethyl)-, (3bS,4aR)-
- CAS:2189684-58-8
- MF:C40H34ClF10N7O7S3
- Structure:
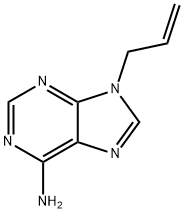
- Chemical Name:NSC 77154
- CAS:4121-39-5
- MF:C8H9N5
- Structure:
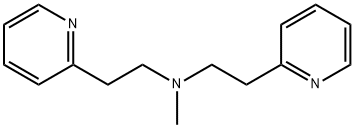
- Chemical Name:N-Methyl-N,N-bis(2-pyridylethyl)amine
- CAS:5452-87-9
- MF:C15H19N3
- Chemical Name:Olodaterol Impurity 26
- CAS:
- MF:
- Structure:
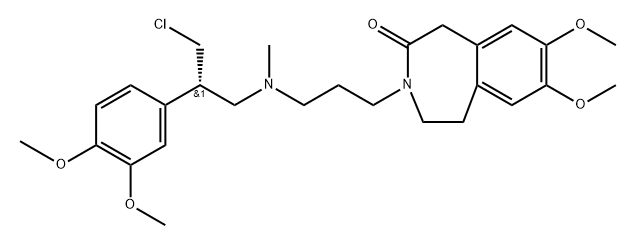
- Chemical Name:2H-3-Benzazepin-2-one, 3-[3-[[(2S)-3-chloro-2-(3,4-dimethoxyphenyl)propyl]methylamino]propyl]-1,3,4,5-tetrahydro-7,8-dimethoxy-
- CAS:2768438-46-4
- MF:C27H37ClN2O5
- Chemical Name:Ethacridine Impurity 5
- CAS:869002-00-6
- MF:
- Chemical Name:Olodaterol Impurity 29
- CAS:
- MF:
- Chemical Name: Elagolix Impurity 40
- CAS:
- MF:
- Structure:

- Chemical Name:1-[4-(2-AZEPAN-1-YL-ETHOXY)-BENZYL]-5-BENZYLOXY-2-(4-BENZYLOXY-PHENYL)-3-METHYL-1H-INDOLE
- CAS:198480-21-6
- MF:C44H46N2O3
- Structure:
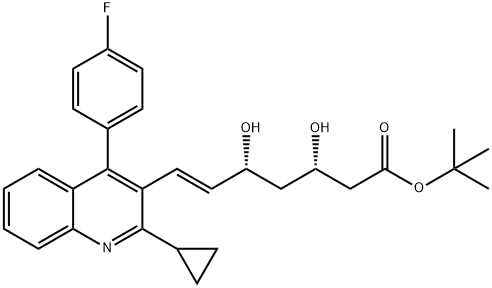
- Chemical Name:6-Heptenoic acid, 7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]-3,5-dihydroxy-, 1,1-dimethylethyl ester, (3S,5R,6E)-
- CAS:2273873-66-6
- MF:C29H32FNO4
- Structure:
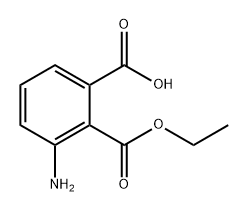
- Chemical Name:1,2-Benzenedicarboxylic acid, 3-amino-, 2-ethyl ester
- CAS:1822756-03-5
- MF:C10H11NO4
- Structure:
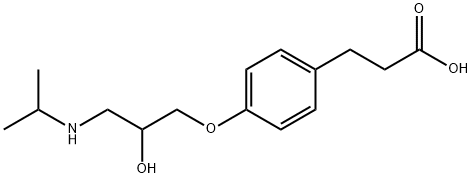
- Chemical Name:ALS 8123
- CAS:81148-15-4
- MF:C15H23NO4
- Structure:
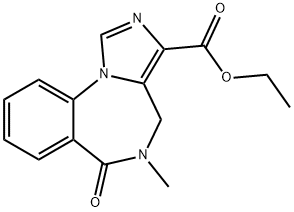
- Chemical Name:Ro 14-7437
- CAS:78756-03-3
- MF:C15H15N3O3
- Structure:
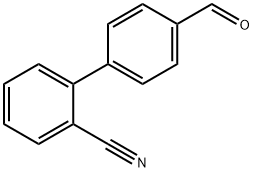
- Chemical Name:4-(2-Cyanophenyl)benzaldehyde
- CAS:135689-93-9
- MF:C14H9NO
- Structure:
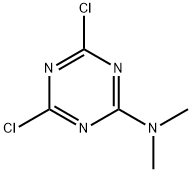
- Chemical Name:2-(N,N-DIETHYLAMINO)-4,6-DICHLOROTRIAZINE
- CAS:2401-64-1
- MF:C5H6Cl2N4
- Structure:
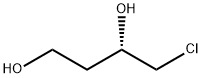
- Chemical Name:(S)-4-Chloro-1,3-butanediol
- CAS:139013-68-6
- MF:C4H9ClO2
- Structure:
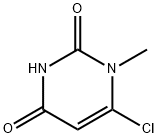
- Chemical Name:6-CHLORO-1-METHYLURACIL
- CAS:31737-09-4
- MF:C5H5ClN2O2
- Structure:
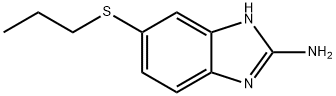
- Chemical Name:5-(Propylthio)-1H-benzimidazol-2-amine
- CAS:80983-36-4
- MF:C10H13N3S
- Chemical Name:Bumetanide iMpurity F
- CAS:
- MF:
- Structure:
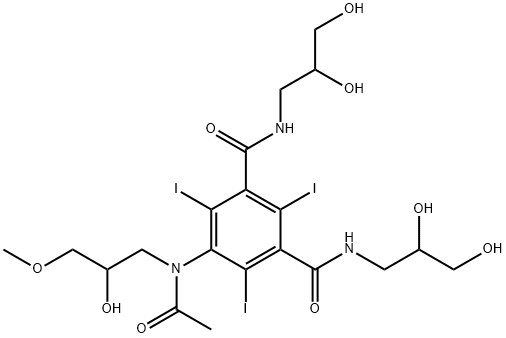
- Chemical Name:IODIXANOL RELATED COMPOUND D (50 MG) (5-[ACETYL(2-HYDROXY-3-METHYLPROPYL)AMINO]-N,N'-BIS(2,3-DIHYDROXYPROPYL)2,4,6-TRIIODO-1,3-BENZE-NEDICARBOXAMIDE)
- CAS:89797-00-2
- MF:C20H28I3N3O9
- Structure:
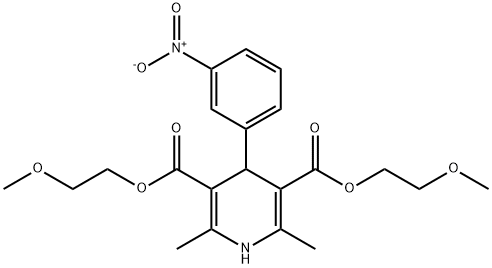
- Chemical Name:NIMODIPINE RELATED COMPOUND B (50 MG) (BIS(2-METHOXYETHYL) 2,6-DIMETHYL-4-(3-NITROPHE-NYL)-1,4-DIHYDROPYRIDINE-3,5-DICARBOXYLATE) (AS)
- CAS:70172-96-2
- MF:C21H26N2O8
- Structure:
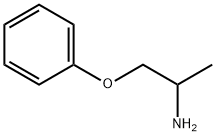
- Chemical Name:1-METHYL-2-PHENOXYETHYLAMINE
- CAS:35205-54-0
- MF:C9H13NO
- Structure:
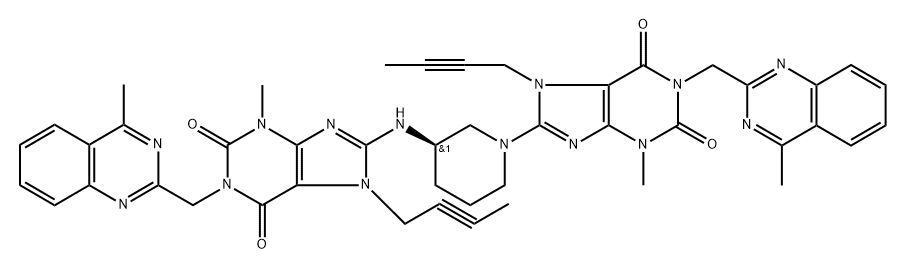
- Chemical Name:N-Depiperidin-3-amine Linagliptin Dimer
- CAS:2253964-98-4
- MF:C45H44N14O4
- Structure:
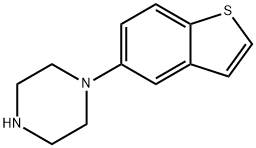
- Chemical Name:Brexpiprazole Impurity 76
- CAS:433303-94-7
- MF:C12H14N2S
- Structure:
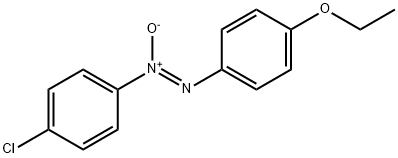
- Chemical Name:Phenacetin Impurity 3
- CAS:24176-84-9
- MF:C14H13ClN2O2
- Structure:
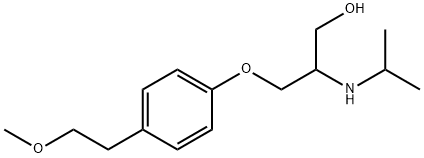
- Chemical Name:2-(isopropylamino)-3-(4-(2-methoxyethyl)phenoxy)propan-1-ol
- CAS:109632-11-3
- MF:C15H25NO3
- Chemical Name:Pabuxilibu impurities
- CAS:3079368-27-4
- MF:
- Chemical Name:ERYTHROMYCIN IMPURITY G
- CAS:
- MF:
- Chemical Name:Amylmetacresol EP impurity E
- CAS:
- MF:
- Structure:
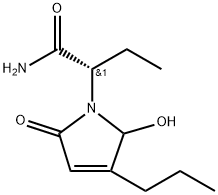
- Chemical Name:(2S)-2-(2,5-dioxo-3-propylpyrrolidin-1-yl)butanamide
- CAS:2232201-72-6
- MF:C11H18N2O3
- Structure:
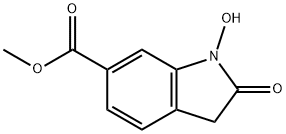
- Chemical Name:methyl 1-hydroxy-2-oxoindoline-6-carboxylate
- CAS:1895918-24-7
- MF:C10H9NO4
- Chemical Name:Dapagliflozin Impurity (MW: 437Da)
- CAS:
- MF:
- Chemical Name:Cetirizine Impurity BHA
- CAS:
- MF:
- Chemical Name:Macitentandesbromopyrimidine Impurity
- CAS:
- MF:
- Structure:
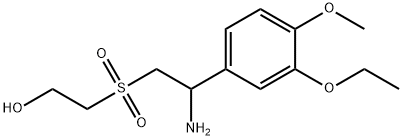
- Chemical Name:Ethanol, 2-[[2-amino-2-(3-ethoxy-4-methoxyphenyl)ethyl]sulfonyl]-
- CAS:2733250-84-3
- MF:C13H21NO5S
- Structure:
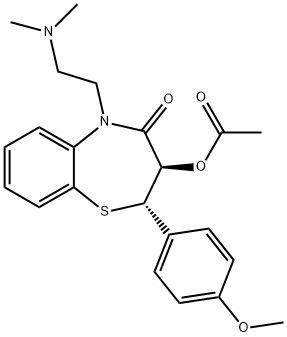
- Chemical Name:1,5-Benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-, (2S-trans)-
- CAS:111188-70-6
- MF:C22H26N2O4S
- Structure:

- Chemical Name:1H-Benzimidazole-2-sulfinicacid(9CI)
- CAS:58536-71-3
- MF:C7H6N2O2S
- Structure:
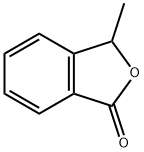
- Chemical Name:1(3H)-Isobenzofuranone,3-methyl-(9CI)
- CAS:3453-64-3
- MF:C9H8O2
- Chemical Name:Oprinone Impurity 5
- CAS:
- MF:
- Chemical Name:Oprinone Impurity 2
- CAS:
- MF:
- Chemical Name:(R)-Cilastatin EP Impurity G
- CAS:
- MF:
- Structure:
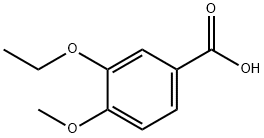
- Chemical Name:3-ETHOXY-4-METHOXYBENZOIC ACID
- CAS:2651-55-0
- MF:C10H12O4
- Structure:
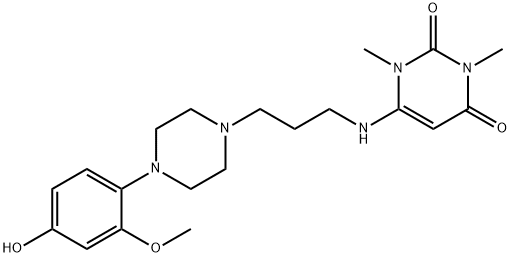
- Chemical Name:4-hydroxyurapidil
- CAS:88733-12-4
- MF:C20H29N5O4
- Structure:
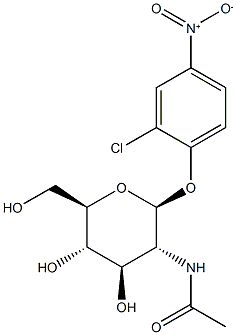
- Chemical Name:2-chloro-4-nitrophenyl-N-acetylglucosaminide
- CAS:103614-82-0
- MF:C14H17ClN2O8
- Chemical Name:Imimidistine impurity D
- CAS:
- MF:
- Structure:
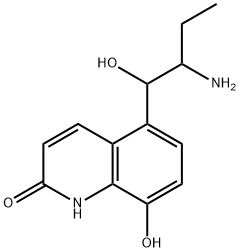
- Chemical Name:desisopropylprocaterol
- CAS:63483-95-4
- MF:C13H16N2O3
- Structure:
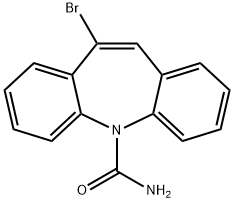
- Chemical Name:10-bromocarbamazepine
- CAS:59690-97-0
- MF:C15H11BrN2O
- Structure:
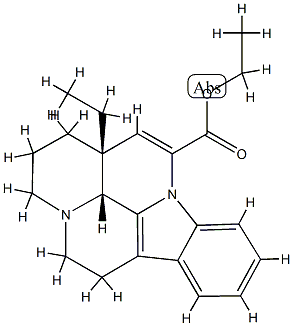
- Chemical Name:ethyl apovincaminate
- CAS:42971-12-0
- MF:C22H26N2O2
- Structure:
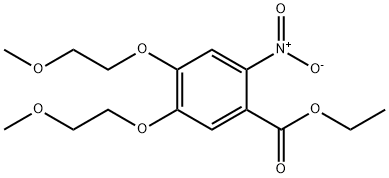
- Chemical Name:Ethyl 4,5-bis(2-methoxyethoxy)-2-nitrobenzoate
- CAS:179688-26-7
- MF:C15H21NO8
- Structure:
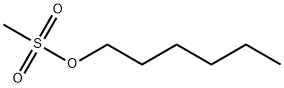
- Chemical Name:Hexyl methanesulfonate
- CAS:16156-50-6
- MF:C7H16O3S
- Structure:
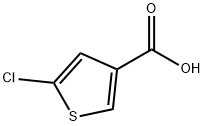
- Chemical Name:5-CHLOROTHIOPHENE-3-CARBOXYLIC ACID
- CAS:36157-42-3
- MF:C5H3ClO2S
- Structure:
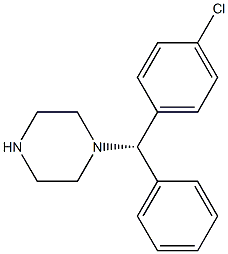
- Chemical Name:(S)-(+)-1-[(4-Chlorophenyl)phenylmethyl]piperazine
- CAS:439858-21-6
- MF:C17H19ClN2
- Structure:
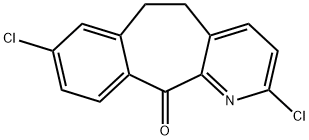
- Chemical Name:2,8-Dichloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-β]pyridin-11-one
- CAS:133330-61-7
- MF:C14H9Cl2NO
- Structure:
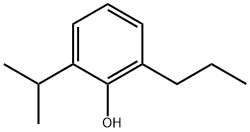
- Chemical Name:2-Isopropyl-6-propylphenol (Propofol Impurity O)
- CAS:74663-48-2
- MF:C12H18O
- Structure:
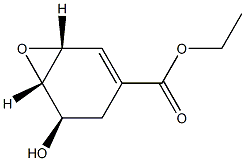
- Chemical Name:(1R,5R,6S)-5-Hydroxy-7-oxabicyclo[4.1.0]hept-2-ene-3-carboxylic Acid Ethyl Ester
- CAS:1476028-68-8
- MF:C9H12O4
- Structure:
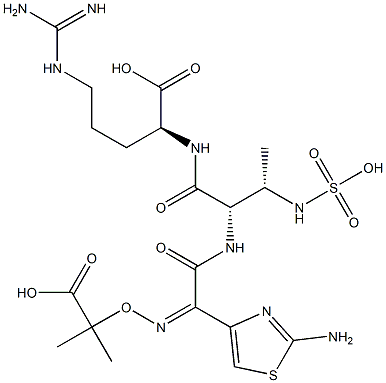
- Chemical Name:(8S,11S,Z)-5-(2-Aminothiazol-4-yl)-11-(3-guanidinopropyl)-2,2-dimethyl-6,9-dioxo-8-((S)-1-(sulfoamino)ethyl)-3-oxa-4,7,10-triazadodec-4-ene-1,12-dioic Acid
- CAS:
- MF:C19H31N9O10S2
- Chemical Name:Brepperazole impurity
- CAS:
- MF:
- Structure:
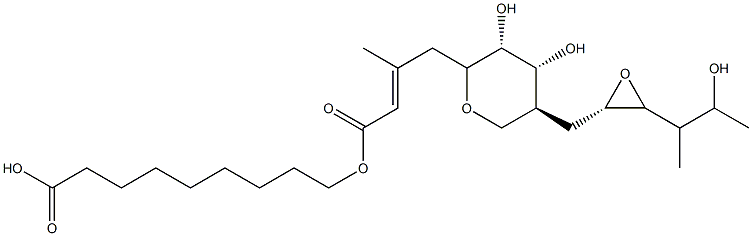
- Chemical Name:Mupirocin iMpurity 4
- CAS:
- MF:C26H44O9
- Structure:
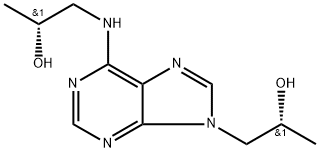
- Chemical Name:Tenofovirdisoproxil Impurity C
- CAS:1878175-82-6
- MF:C11H17N5O2
- Structure:
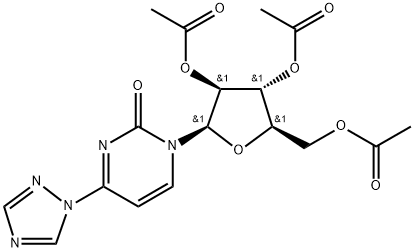
- Chemical Name:Cytarabine Impurity 13
- CAS:82855-62-7
- MF:C17H19N5O8
- Structure:
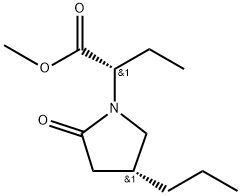
- Chemical Name:2052297-80-8
- CAS:2052297-80-8
- MF:C12H21NO3
- Chemical Name:Pramipexole Impurity L
- CAS:
- MF:
- Structure:
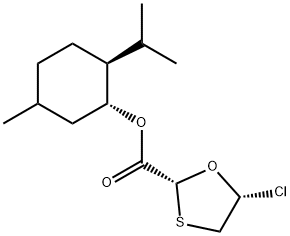
- Chemical Name:1020151-61-4
- CAS:1020151-61-4
- MF:C14H23ClO3S
- Chemical Name:Nicergoline Impurity M
- CAS:
- MF:
- Chemical Name:Apixaban Impurity 48
- CAS:
- MF:
- Structure:
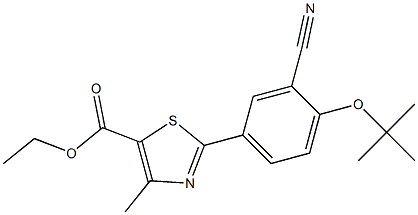
- Chemical Name:ethyl 2-(4-(tert-butoxy)-3-cyanophenyl)-4-methylthiazole-5- carboxylate
- CAS:
- MF:C18H20N2O3S
- Structure:
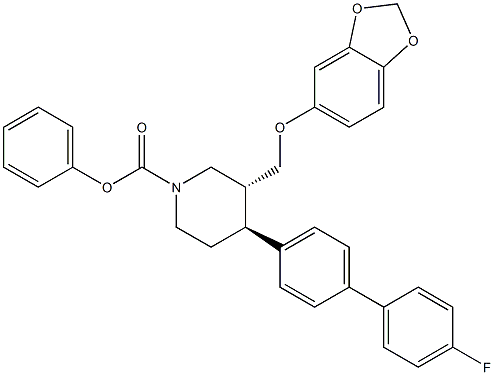
- Chemical Name:(trans)-phenyl 3-((benzo[d][1,3]dioxol-5-yloxy)methyl)-4-(4'-fluoro- [1,1'-biphenyl]-4-yl)piperidine-1-carboxylate
- CAS:
- MF:C32H28FNO5
- Chemical Name:Moxifloxacin Impurity 9
- CAS:
- MF:
- Chemical Name:Ticagrelor Impurity 10
- CAS:
- MF:
- Chemical Name:Tofacitinib Impurity O
- CAS:
- MF:
- Structure:
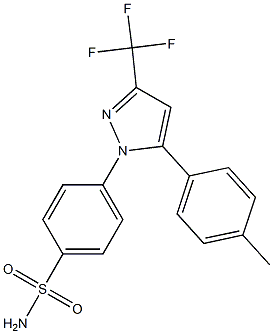
- Chemical Name:Celecoxib Impurity 5
- CAS:
- MF:C17H14F3N3O2S
- Chemical Name:Prasugrel Impurity 10
- CAS:
- MF:
- Structure:
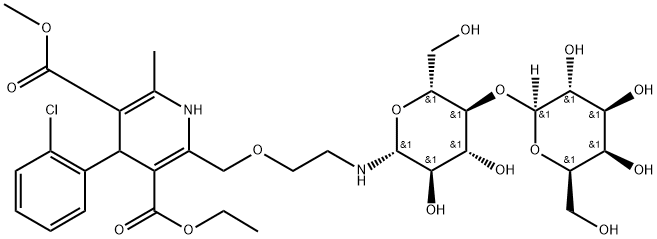
- Chemical Name:Amlodipine N-Lactoside
- CAS:749864-27-5
- MF:C32H45ClN2O15
- Chemical Name:Sugammadex Impurity 19
- CAS:2379877-89-9
- MF:
- Chemical Name:Apixaban Impurity 29
- CAS:
- MF:
- Chemical Name:Alogliptin Impurity 22
- CAS:
- MF:
- Structure:
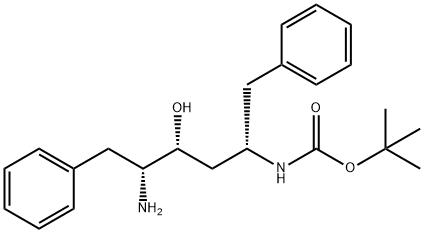
- Chemical Name:Ritonavir Impurity
- CAS:1414933-89-3
- MF:C23H32N2O3
- Chemical Name:Riociguat Impurity 7
- CAS:
- MF:
- Structure:
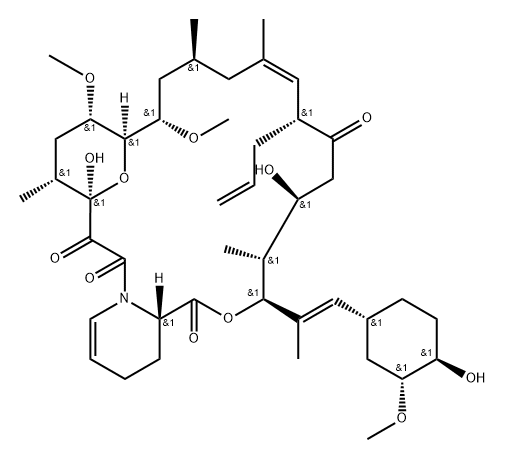
- Chemical Name:Tacrolimus Impurity 5
- CAS:161861-18-3
- MF:C44H67NO12
- Structure:
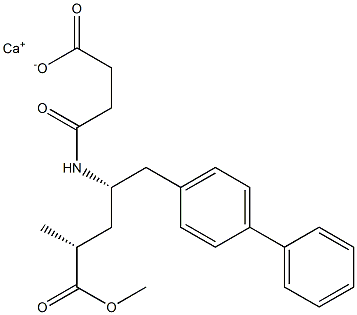
- Chemical Name:monocalciummono(4-(((2S,4R)-1-([1,1'-biphenyl]-4-yl)-5- methoxy-4-methyl-5-oxopentan-2-yl)amino)-4-oxobutanoate)
- CAS:
- MF:C23H26CaNO5
- Chemical Name:Aprepitant iMpurity 38
- CAS:
- MF:
- Chemical Name:Aprepitant iMpurity 41
- CAS:
- MF:
- Chemical Name:(E)-3'-(2-(1-(3,4-dimethylphenyl)
- CAS:
- MF:
- Structure:
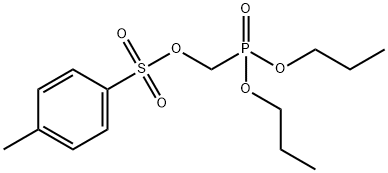
- Chemical Name:Tenofovir
- CAS:34956-22-4
- MF:C14H23O6PS
- Chemical Name:Afatinib Impurity YHQ
- CAS:
- MF:
- Structure:
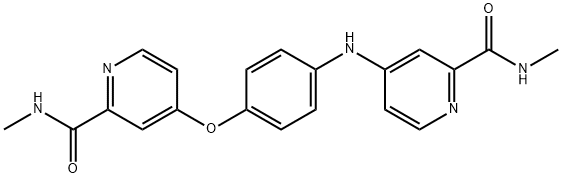
- Chemical Name:Sorafenib impurity 24/N-Methyl-4-[4-[[2-[(methylamino)carbonyl]-4-pyridinyl]amino]phenoxy]-2-pyridinecarboxamide
- CAS:2004659-83-8
- MF:C20H19N5O3
- Structure:
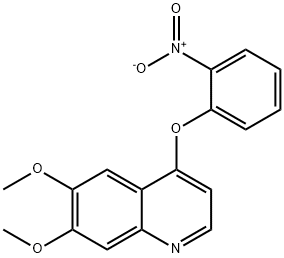
- Chemical Name:Cabozantinib Impurity 49
- CAS:190728-26-8
- MF:C17H14N2O5
- Chemical Name:Landiolol impurity S
- CAS:
- MF:
- Structure:
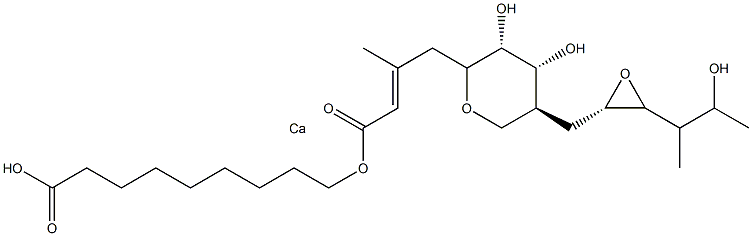
- Chemical Name:Mupirocin Calcium EP Impurity G
- CAS:
- MF:C26H44CaO9
- Structure:
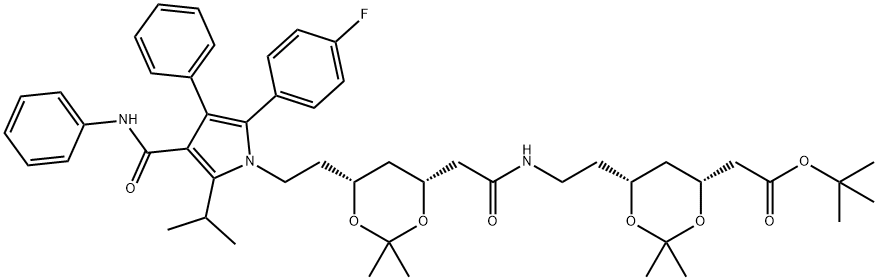
- Chemical Name:Atorvastatin Impurity 40
- CAS:1116118-82-1
- MF:C50H64FN3O8
- Chemical Name:Hyoscine Butylbromide EP Impurity F
- CAS:
- MF:
- Chemical Name:Tocopherol EP Impurity D
- CAS:
- MF:
- Chemical Name:Org246653-1(RRT0.35)
- CAS:
- MF:
- Structure:
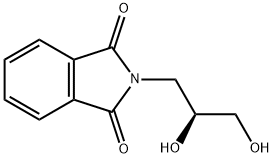
- Chemical Name:1H-Isoindole-1,3(2H)-dione, 2-[(2S)-2,3-dihydroxypropyl]-
- CAS:119835-88-0
- MF:C11H11NO4
- Chemical Name:Pramipexole 828 Impurity
- CAS:
- MF:
- Chemical Name:E-Ceftazidime Oxide Impurity
- CAS:
- MF:
- Chemical Name:Cefuroxime Impurity D Δ3-Isomer
- CAS:
- MF:
- Chemical Name:Ampicillin Open-Ring Formylation Impurity
- CAS:
- MF:
- Structure:
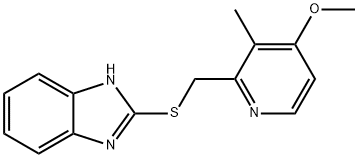
- Chemical Name:2-[(4-METHOXY-3-METHYL-2-PYRIDINYL)-METHYLTHIO]-BENZIMIDAZOLE
- CAS:102804-82-0
- MF:C15H15N3OS
- Structure:
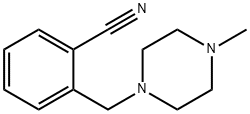
- Chemical Name:2-[(4-METHYLPIPERAZIN-1-YL)METHYL]BENZONITRILE
- CAS:864069-00-1
- MF:C13H17N3