Potassium superoxide
12030-88-5 Basic informationMore
- Product Name:Potassium superoxide
- Synonyms: PotassiuM Superoxide, Uncatalyzed Granules PotassiuM Superoxide, Uncatalyzed Granules, 3/8 to 1/4 in. Potassium dioxide chunks, 5-10 mm burntpotash cacinedpotash K(O2) Potassium oxide super Potassium superoxide ko2
- CAS:12030-88-5
- MF:KO2*
- MW:71.1
- EINECS:234-746-5
- Mol File:12030-88-5.mol
-
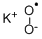
USE
Potassium superoxide is a powerful oxidizer. Forms on the surface of potassium metal, solid or molten, that is exposed to the air. Attempts to extinguish burning potassium with powdered graphite has resulted in violent explosions [Chem. Abstr. 63:424. 1965]. Highly oxidized potassium metal was dropped into a dish of ethyl alcohol, an immediate explosion shattered the dish. Potassium superoxide was considered the cause of the reaction [Health and Safety Inf. 251. 1967]. Potassium superoxide should not be added to pure organic materials (hydrocarbons), as ignition and violent explosion may occur. Oxidation of arsenic, antimony, copper, potassium, tin, or zinc proceeds with incandescence, [Mellor, 1941, Vol. 2, 493]. Interaction between the superoxide and diselenium dichloride is violent, [Mellor, 1947, Vol. 10, 897].
Browse by Nationality 12030-88-5 Suppliers >Global suppliers
Please select the suppliers
Recommend You Select Member Companies
Recommend You Select Member Companies