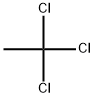
1,1,1-Trichloroethane
71-55-6 Basic informationMore
- Product Name:1,1,1-Trichloroethane
- Synonyms: 1,1,1-Trichloroethane in dimethyl sulfoxide 1,1,1-Trichloroethane Standard Residual Solvent Class 1 - 1,1,1-Trichloroethane 1,1,1-Trichloroethane in Dimethyl Sulfoxide, USP 467 Standard 1,1,1-trichloroethane(tcea) 1,1,1-Trichloroethane-surfactants-butane-propane 1,1,1-Tricloroetano Aerothene TT
- CAS:71-55-6
- MF:C2H3Cl3
- MW:133.4
- EINECS:200-756-3
- Mol File:71-55-6.mol
-
USE
1,1,1-Trichloroethane decomposes in the presence of chemically active metals. This includes aluminum, magnesium and their alloys. 1,1,1-Trichloroethane will react violently with dinitrogen tetraoxide, oxygen, liquid oxygen, sodium and sodium-potassium alloys. 1,1,1-Trichloroethane will also react violently with acetone, zinc and nitrates. 1,1,1-Trichloroethane can react with sodium hydroxide. 1,1,1-Trichloroethane is incompatible with strong oxidizers and strong bases. Mixtures with potassium or its alloys are shock-sensitive and may explode on light impact. 1,1,1-Trichloroethane can react with an aqueous suspension of calcium hydroxide, and with chlorine in sunlight. 1,1,1-Trichloroethane will attack some forms of plastics, rubber and coatings. Upon contact with hot metal or on exposure to ultraviolet radiation, 1,1,1-Trichloroethane will decompose to form irritant gases. A cobalt/molybdenum-alumina catalyst will generate a substantial exotherm on contact with its vapor at ambient temperatures. Hazardous reactions also occur with (aluminum oxide + heavy metals). .
Browse by Nationality 71-55-6 Suppliers >Global suppliers
Please select the suppliers
Recommend You Select Member Companies
Recommend You Select Member Companies