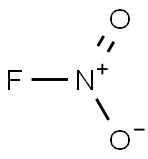
nitryl fluoride
10022-50-1 Basic informationMore
- Product Name:nitryl fluoride
- Synonyms: nitryl fluoride Nitro fluoride Nitryl fluoride ((NO2)F)
- CAS:10022-50-1
- MF:FNO2
- MW:65
- EINECS:2330210
- Mol File:10022-50-1.mol
-
USE
Nitryl fluoride is a strong oxidizing agent. Most reactions are similar to those of nitryl chloride, NO2Cl (See Nitryl Chloride Reactions). Nitryl fluoride hydrolyzes rapidly in water forming nitric acid and hydrofluoric acid:<br />
NO2F + H2O → HNO3 + HF<br />
Reaction with ethanol produces ethyl nitrate:<br />
NO2F + C2H5OH → C2H5NO3 + HF<br />
Reactions with reducing agents can be explosive. The compound attacks most metals almost as vigorously as fluorine. It spontaneously ignites boron, silicon, phosphorus, arsenic, antimony, and iodine at ordinary temperatures.<br />
Nitryl fluoride can add a nitrate group to many organics forming their nitro derivatives:<br />
C6H6 + NO2F → C6H5NO2 + HF
Please select the suppliers
Recommend You Select Member Companies
Recommend You Select Member Companies
- Company Name:Shaanxi Didu New Materials Co. Ltd
- Tel:+86-89586680 +86-13289823923
- Products Intro:Product Name:Nitrylfluoride;10022-50-1
CAS:10022-50-1
Purity:0.99 Package:25KG,200L - CB Index:58
- Related Information:Catalog(8670)