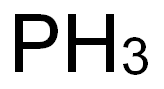White or yellow white phosphorus is a yellow waxy or colourless, transparent, volatile crystalline
solid, waxy appearance with a garlic-like odour. On exposure to light, it darkens and
ignites in air. It is also called yellow phosphorus colour because of impurities. White phosphorus
does not occur naturally but is manufactured from phosphate rocks. It is insoluble
in water, slightly soluble in benzene, ethanol, and chloroform, and is soluble in carbon disulphide. White phosphorus reacts rapidly with oxygen, easily catching fire at temperatures
10°C–15°C above room temperature. White phosphorus is used by the military in various
types of ammunition and to produce smoke for concealing troop movements and identifying
targets. It is also used by industry to produce phosphoric acid and other chemicals for use
in fertilisers, food additives, and cleaning compounds. Small amounts of white phosphorus
were used in the past in pesticides and fireworks.White phosphorus is used mainly for producing phosphoric acid and other chemicals.
These chemicals are used to make fertilisers, additives in foods and drinks, cleaning compounds,
and other products. In the military, white phosphorus is used in ammunitions such
as mortar and artillery shells, and grenades.