ITP is an autoimmune disease in which antiplatelet antibodies accelerate the destruction of platelets. In addition, platelet production can be
impaired because the antiplatelet antibodies can also damage megakaryocytes. Although the thrombocytopenia of ITP can be severe, signs of
bleeding are usually only minor.
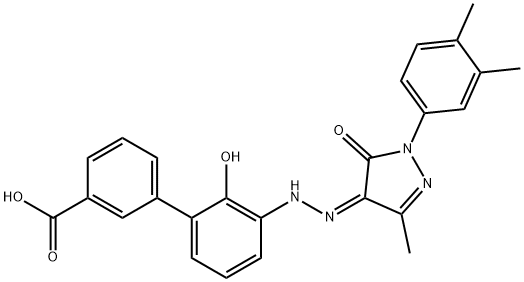
Eltrombopag is an oral, small-molecule, nonpeptide TPO-receptor agonist. It initiates TPO-receptor signaling by interacting with the
transmembrane domain of the receptor, thereby inducing proliferation
and differentiation of cells in the megakaryocytic lineage. Eltrombopag is
the second TPO-receptor agonist to reach the market behind romiplostim
(Nplate), a recombinant fusion protein launched in 2008. Both drugs are
specifically indicated for the treatment of thrombocytopenia in patients
with chronic ITP who have had an insufficient response to corticosteroids,
immunoglobulins, or splenectomy. Eltrombopag is supplied as the ethanolamine salt (also known as eltrombopag olamine).
The most common adverse reactions associated with eltrombopag were nausea, vomiting, mennorrhagia, myalgia, paresthesia and cataracts. Eltrombopag is derived in five synthetic steps starting from 2-bromo-6-nitroanisole, via Suzuki coupling reaction with 3-carboxyphenylboronic acid to a biphenyl intermediate, followed by cleavage of the methyl ether moiety with hydrobromic acid, and reduction of the nitro group to an amino group under catalytic hydrogenation conditions. Subsequently, the amino group is diazotized with sodium nitrite and hydrochloric acid, and the diazonium intermediate is condensed with 1-(3,4-dimethylphenyl)-3-methyl-2,5-dihydro-1H-pyrazol-5-one to produce eltrombopag.