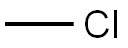Methyl chloride is a colorless, flammable gas
with a faintly sweet, nonirritating odor at room
temperature. It is shipped as a transparent liquid
under its vapor pressure of about 59 psig at
70°F (407 kPa at 21.1℃).
Methyl chloride burns feebly in air, but forms
mixtures with air that can be explosive within its
flammability range.
Dry methyl chloride is very stable at normal
temperatures and in contact with air. In the
presence of moisture, it hydrolyzes slowly,
which results in the formation of corrosive hydrochloric
acid. At temperatures above 700°F
(371℃), methyl chloride may decompose into
toxic end-products (hydrochloric acid, phosgene,
chlorine, and carbon monoxide). It is
slightly soluble in water and very soluble in
alcohol, mineral oils, chloroform, and most organic
liquids.