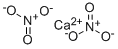Calcium nitrate has the molecular formula of
Ca(NO3)2 and the molecular weight of 164.0935 g/mol.
Its CAS number is 10124-37-5. Calcium nitrate may be
prepared by the reaction of nitric acid with calcium
carbonate or calcium sulfide:
CaCO3 + 2HNO3 Ca(NO3)2 + CO +H2O
CaS + 2HNO3 Ca(NO3)2 +H2S
It is very soluble and forms a tetrahydrate, Ca(NO3)
2·4H2O if the solution is evaporated to dryness.
The CAS number of the anhydrate is 10124-37-5 while
that of the tetrahydrate is 13477-34-4. As with the beryllium
nitrate salts, variation of the nitric acid concentration
affects the type of hydrate produced. The
trihydrate is known (CAS number 10124-37-5) but not
the monohydrate. The anhydrate is a white cubic crystal
that is hygroscopic; its density is 2.504 g/cm3 and it
melts at 561°C. This salt is highly soluble in water and
also dissolves in alcohols and acetone. The tetrahydrate
melts in its own waters of hydration at 42.7°C and loses
4H2O molecules at 132°C. It decomposes to form
nitrogen oxides and CaO similar to the behavior of the
other alkaline earth nitrates. The anhydrous salt is
soluble at 121.2 g/100 ml at 20°C and 271.2 g/100 ml
at 40°C.