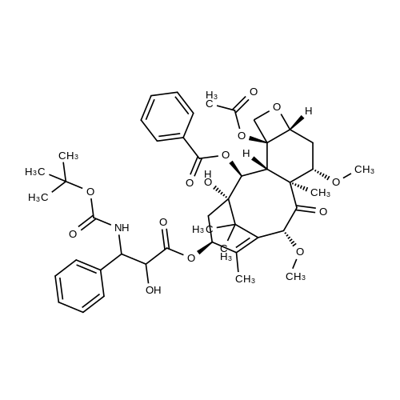
In June 2010, the U.S. FDA approved cabazitaxel (also referred to as
XRP6258 and RPR 116258A) in combination with the steroid prednisone
for the treatment of metastatic Castration-Resistant Prostate Cancer
(mCRPC) for patients who were previously treated with a docetaxelcontaining
regimen for late-stage disease.
Cabazitaxel is a semisynthetic analog of the
natural product taxol, which is isolated from the bark of the yew tree.
Cabazitaxel is a microtubule inhibitor that binds to the taxol-binding site of
tubulin. Similar to other tubulin inhibitors of the taxol class, cabazitaxel
inhibits microtubule disassembly resulting in mitotic blockade and cell
death. Docetaxel, also a semisynthetic taxol analog, was approved by the
FDA for the treatment of mCRPC in 2004. However, docetaxel is a substrate
for P-gp, which is thought to contribute to the constitutive and acquired
resistance of cancer cells to taxanes. Cabazitaxel has poor affinity for P-gp
and showed antitumor activity in preclinical in vitro studies and in vivo
tumor models that overexpress this protein. Cabazitaxel is synthesized on
a commercial scale from 10-deacetylbaccatin .