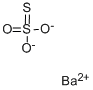Barium thiosulfate has the molecular formula of
BaS2O3 and the molecular weight of 249.4602 g/mol. It
can be prepared by the reaction of sodium thiosulfate
in water with the chloride of barium:
BaCl2+Na2S2O3 BaS2O3+2NaCl
It is not very soluble in water (0.25 g/100 ml at 15°C)
so that the solution does not need to be evaporated at
a low temperature to crystallize the product. A monohydrate
is the result. BaS2O3·H2O has the CAS number of
35112-53-9. It is insoluble in ethanol. It is a white, crystalline,
water-insoluble, poisonous solid. It loses the water
of hydration at 110°C and decomposes at 228°C to form
the sulfate, sulfur and SO2.
BaS2O3·H2O is used chiefly in the manufacture of
explosives, matches, paints, and varnishes. However,the demand for this chemical is not substantial and it
is classified as a “rare” chemical.