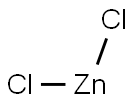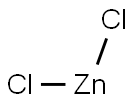Zinc(II) chloride is used as a catalyst, as a dehydrating and condensing agent, soldering flux and metal etchant. It is also employed in preserving anatomical specimens, wood preservatives, deodorant, disinfecting and embalming materials. It is employed as a mordant in printing and dyeing materials, and in the vulcanizing processes of fiber and rubber. It is useful as an electrolyte in dry cell batteries, in metal industry, in galvanizing iron and as an electrolyte for electroplating. It is useful in an organic synthesis, for example in the Friedel-crafts acylation, Fisher indole synthesis, Lucas reagent (zinc chloride with HCl), activation of allylic/benzylic halides towards reaction with olefins or with sodium cyanoborohydride catalyzed reduction of halides, organozinc reagents for use in Negishi coupling, and aldol condensation reactions.