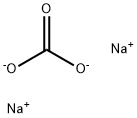
Sodium carbonate (molecular formula: Na2CO3), is the water soluble sodium salt of carbonate. The pure product appears as a while, odorless powder with a strong alkaline taste. It has high hygroscopicity. It can be easily dissolved in water to form an aqueous solution with moderate alkalinity. Sodium carbonate has wide applications in various kinds of fields around the world. One of most important application of sodium carbonate is for the manufacturing of glass. Based on statistics information, about half of the total production of sodium carbonate is used for the manufacturing of glass. During the production of glass, sodium carbonate acts as a flux in the melting of silica. In addition, as a strong chemical base, it is used in the manufacturing of pulp and paper, textiles, drinking water, soaps and detergents and as a drain cleaner. In addition, it can also be used for tissue digestion, dissolving amphoteric metals and compounds, food preparation as well as acting as a cleaning agent. There are generally two ways for the production of sodium carbonate. One is through the reactions between sodium chloride and calcium carbonate (via the ammonia soda (Solvay) process). The other is from sodium carbonate and hydrogencarbonate ores (trona and nahcolite).