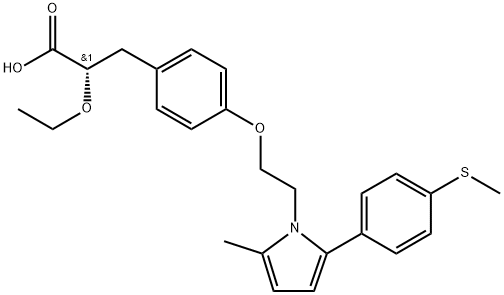
Saroglitazar (also known as ZYH1) was approved for use by the Drug Controller General of India for the treatment of diabetic dyslipidemia and hypertriglyceridemia that is not controlled by statin therapy. Saroglitazar is the first approved agent with dual PPAR agonist activity, with EC50s of 0.00065 and 3 nM, respectively, for the α- and γ-PPAR isoforms in HepG2 cells. The medicinal chemistry program has not been described in the scientific literature, but numerous substituents on the pyrrole ring are described in the saroglitazar patent. These substituted pyrroles were synthesized via ethanolamine condensation with substituted diketones and subsequent activation of the hydroxyethylpyrrole for phenolic etherification with (S)-2- ethoxy-3-(4-hydroxyphenyl)propanoic acid. The efficacy of saroglitazar was evaluated in db/db mice (genetically diabetic animals that lack the leptin receptor), wherein it produced a 17–55% reduction in serum triglycerides (TGs) after 12 days of administration of saroglitazar (0.01–3 mg/kg).