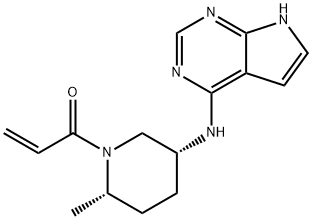
Ritlecitinib, a dual JAK3/TEC inhibitor, was granted FDA breakthrough status for treating alopecia areata (autoimmune-induced hair loss) in September 2018. Promising phase 3 trial results in alopecia areata were reported by Pfizer in August 2021, placing it in competition with Lilly/Incyte’s Olumiant. It is also used in clinical studies to treat vitiligo, rheumatoid arthritis, Crohn’s disease, and ulcerative colitis. Ritlecitinib achieved high JAK3 selectivity via covalent interaction with a unique cysteine residue (Cys909) in the catalytic domain.