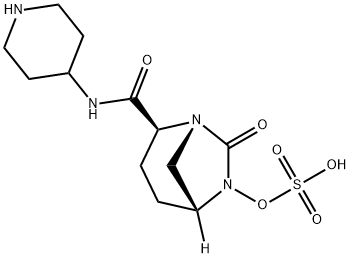
Relebactam (formerly known as MK-7655) is a novel,intravenous,class A and class C B-lactamase inhibitor and is currently under evaluation in combination with imipenem/cilastatin for the treatment of resistant Gram-negative infections.In vitro studies demonstrated that relebactam restored imipenem's activity against KPC-producing Enterobacteriacae,lowering imipenem MICs from 16-64 to 0.12-1mg/L at a concentration of 4 mg/L. Moreover, relebactam is able to lower imipenem MICs for P. aeruginosa,particularly in strains with depressed OprD expression and increased AmpC expression.Conversely, the addition of relebactam to imipenem does not seem to provide any adjunctive benefit against A.baumanii or S.maltophilia or against MBL-producing Enterobacteriacae.A non-inferiority, Phase 3 trial evaluating the efficacy and safety of imipenem/relebactam compared to piperacillin/tazobactam for the treatment of HAP and VAP(ClinicalTrials.gov Identifier: NCT02493764) is currently recruiting. A Phase 3 study evaluating the efficacy and safety of imipenem/relebactam (200/100-500/250 mg depending on renal function) compared to colistimethate sodium plus imipenem/cilastatin for the treatment of imipenem-resistant bacterial infections,including HAP, VAP, cIAIs and cUTIs, has recently been completed and results are pending(ClinicalTrials.gov Identifier: NCTO2452047).In Phase 2 trials, imipenem/relebactam was well tolerated, with diarrhea, nausea, vomiting and headache being the most commonly reported adverse events.