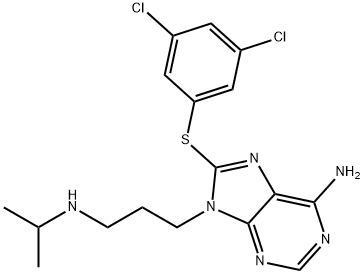
Glucose-regulated protein 94 (Grp94) is a stress-inducible molecular chaperone belonging to the heat shock protein (Hsp) family and, as a result, is also known as Hsp90B1. Grp94 is essential for the processing of proteins that have been implicated in tumorigenesis, including insulin-like growth factor 1 (IGF-1), Toll-like receptors (TLRs), and integrins. It also functions in antigen presentation, and its secreted form can elicit innate and adaptive immune responses. PU-WS13 is a cell-permeable inhibitor of Grp94 (EC50 = 220 nM). It displays selectivity for Grp94 over the related Hsps Trap-1, Hsp90α, and Hsp90β. PU-WS13 blocks the proliferation of HER2 over-expressing SK-BR-3 cells in the sub-G1 phase and induces apoptosis, but doesn’t significantly affect the survival of normal cells. It induces both apoptosis and necrosis in multiple myeloma cells but does not induce death in pre-B leukemic cells. Unlike pan-Hsp90 inhibitors, PU-WS13 does not activate a feedback heat-shock response.