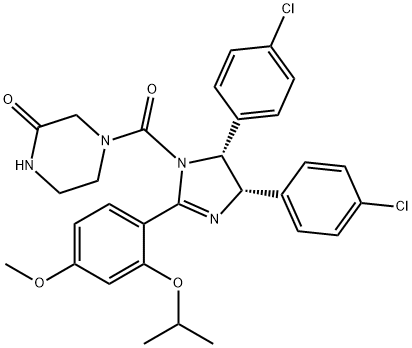
The protein p53, often called the ‘guardian of the genome,’ is a transcription factor that is activated in response to cellular stress (low oxygen levels, heat shock, DNA damage, etc.) and acts to prevent further proliferation of the stressed cell by promoting cell cycle arrest or apoptosis. Its role as a tumor suppressor is evident by the observation that approximately 50% of human tumors have mutated or non-functional p53. Mdm2, a key negative regulator of p53, which is over-expressed in many human tumors, functions by binding to and targeting p53 for proteasomal degradation. Nutlin-3 is a potent inhibitor of p53-Mdm2 interaction. (?)-Nutlin-3 is arbitrarily referred to as enantiomer a because it appears as the first peak from chiral purification of racemic nutlin-3 and its absolute stereocenter assignment is not known. It potently inhibits Mdm2-p53 binding with an IC50 value of 0.09 μM, induces the expression of p53-regulated genes, and exhibits potent antiproliferative activity in cells with functional p53, but not in cells with mutated p53. (?)-Nutlin-3 inhibits the proliferation of exponentially growing human skin and murine fibroblasts with IC50 values of 2.2 and 1.3 μM, respectively, and induces substantial tumor shrinkage in mice expressing LnCaP, 22Rv1 or MHM cancer cell lines when treated orally with a 200 mg/kg dose twice daily for two weeks.