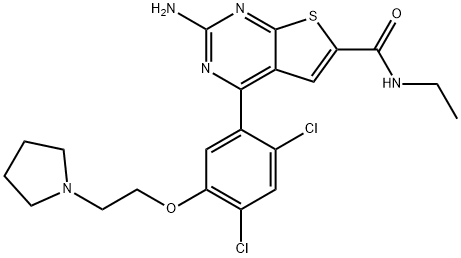
Heat shock protein 90 (Hsp90) isoforms are molecular chaperones that modulate intracellular signaling and protein folding, trafficking, and turnover. Inhibitors of Hsp90, such as geldanamycin , have shown promise as anti-tumor agents. NVP-BEP800 is a potent inhibitor of Hsp90β (IC50 = 58 nM) that is 70-fold less effective against two related Hsps, Grp94 (Hsp90B1) and Trap-1 (Hsp75). It is without effect (IC50 > 10 μM) against a panel of twenty kinases. NVP-BEP800 causes p23/Hsp90 dissociation and client protein degradation, which leads to growth inhibition (GI50s from 53 to 190 nM) or induction of cell death in cancer cell lines. It is orally available and has antitumor effects in human tumor xenografts in mice. Hsp90 inhibitors, including NVP-BEP800, increase the sensitivity of tumor cells to ionizing radiation. They also direct the proteasomal degradation of viral Hsp90 client proteins, including those required for latency and infectivity of Kaposi sarcoma-associated herpes virus.