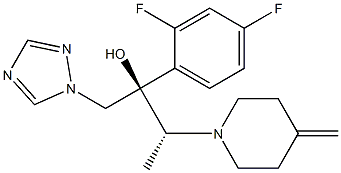
In October 2013, efinaconazole (also known as KP-103) was approved in Canada as a 10% topical solution for the treatment of onychomycosis. Like other azole antifungal agents, efinaconazole acts by disrupting fungal cell membranes through inhibition of sterol 14α-demethylase, an enzyme involved in the biosynthesis of ergosterol, which is a key component of the fungal cell membrane. Efinaconazole has potent antifungal activity against clinical isolates of dermatophytes, including Trichophyton mentagrophyes (MIC80 =0.125 μg/mL) and Trichophyton rubrum (MIC80 =0.25 μg/mL), as well as against Candida and Malassezia species. Unlike other antifungal agents, efinaconazole retains activity in the presence of keratin, indicating that more unbound drug is available at the site of action. Efinaconazole is efficacious in guinea-pig models of fungal infection. Efinaconazole is prepared by reaction of an epoxide intermediate with 4-methylenepiperidine.