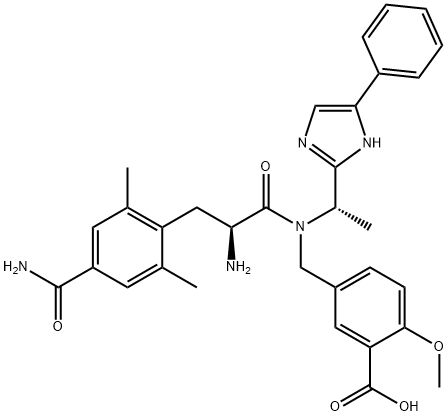
Eluxadoline, originally developed
by Janssen and currently marketed by Allergan (formerly
Actavis), was approved in May 2015 by the FDA for the
treatment of diarrhea-predominant irritable bowel syndrome
(IBS-D). Eluxadoline, an orally dosed agent, employs a
unique mechanism for IBS-D treatment, as it functions
simultaneously as a μ- and κ-opioid receptor agonist and a δ-
opioid receptor antagonist, leading to a first-in-class therapy
for treatment of IBS-D. Specifically, in animal studies,
eluxadoline was found to interact with opioid receptors in the
gut, inhibiting neurogenically mediated secretion and reducing
intestinal contractility. Additionally, the treatment led to a
decrease in stress-induced acceleration of upper GI transit
without causing rebound constipation, earning its mark as
a first-line therapeutic treatment for IBS-D. In two phase III
clinical trials of over 2400 patients with IBS-D, patients taking
eluxadoline showed a greater improvement toward the end
point (≥30% improvement from their baseline IBS-D score on
at least 50% of days treated with eluxadoline) compared to
patients treated with placebo.