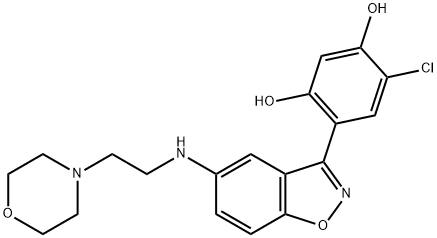
Heat shock protein 90 (Hsp90) is a molecular chaperone that modulates intracellular signaling and protein folding, trafficking, and turnover. Inhibitors of Hsp90, such as geldanamycin, have shown potential as anti-tumor agents. Benzisoxazole Hsp90 Inhibitor binds the N-terminal domain of Hsp90, efficiently displacing geldanamycin from the ATP binding site (IC50 = 30 nM). It also inhibits the proliferation of several cancer cell lines (IC50~ 0.28 μM), promotes the degradation of the Hsp90 client proteins Her-2 and androgen receptor, and has no effect on a variety of kinases.