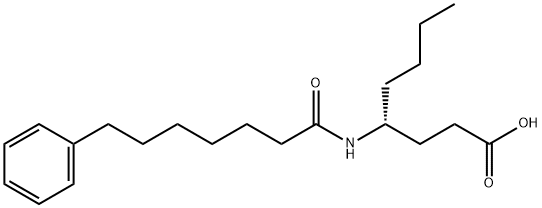
Phospholipase A2 (PLA2) catalyzes the hydrolysis of fatty acids at the sn-2 position of glycerophospholipids, liberating arachidonic acid for subsequent eicosanoid synthesis. Three primary types of PLA2 exist: secretory (sPLA2), calcium-dependent cytosolic (cPLA2), and calcium-independent cytosolic (iPLA2). Of these three enzymes, cPLA2 is the rate-limiting stimulus for release of arachidonic acid whereas sPLA2 amplifies the action of cPLA2 and regulates phagocytosis and foam cell formation. CAY10590, a simple amide based on (R)-γ-norleucine, is a potent and selective inhibitor of sPLA2. It exhibits 95% inhibition (XI(50) = 0.003) of sPLA2 at 0.091 mole fraction without affecting the activities of cPLA2 or iPLA2.