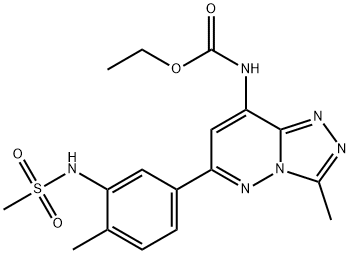
Bromodomains play a key role in many cellular processes, including inflammatory gene expression, mitosis, and viral/host interaction, by controlling the assembly of histone acetylation-dependent chromatin complexes. They serve as “readers” of histone acetylation marks, regulating the transcription of target promoters. For example, the cAMP response element-binding protein binding protein (CREBBP) bromodomain has been shown to modulate the stability and function of the tumor suppressor protein p53. Bromosporine is a non-selective bromodomain inhibitor. At 1 μM, it has been shown to accelerate fluorescence recovery after photobleaching (FRAP) recovery of BRD4 and CREBBP bromodomains in HeLa cells. This compound may be used in determining the biological roles of reader domains as well as a tool for the validation of functional assays. See the Structural Genomics Consortium (SGC) website for more information.