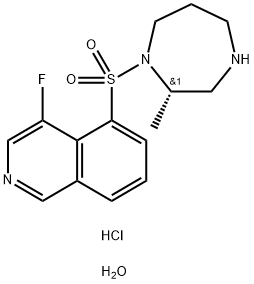
Ripasudil hydrochloride hydrate (Glanatec®) was approved in
Japan in 2014 for the treatment of glaucoma and ocular hypertension.
Originally discovered by D. Western Therapeutics Institute,
Inc. and licensed by the Kowa Company, Ltd, ripasudil
functions as a selective Rho-kinase inhibitor and reduces intraocular
pressure by stimulation of aqueous humour drainage of the
trabecular meshwork. While this recent approval allows
for use of ripasudil as a twice-daily monotherapy treatment when
other drugs cannot be used or are not effective, clinical trials
using ripasudil as a combination therapy with other glaucoma
drugs have shown promising results in the treatment of primary
open-angle glaucoma or ocular hypertension. Currently, the
Kowa Company is also pursuing trials focused on the use of
ripasudil for the treatment of diabetic retinopathy and diabetic
macular edema.