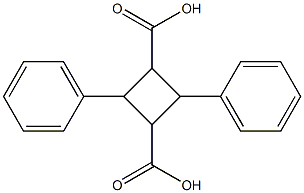
α-Truxillic acid can be formed by the dimerization of two molecules of α-trans-cinnamic acid. It is related to incarvillateine, a natural antinociceptive compound derived from the Asian herb I. sinensis. α-Truxillic acid and some of its derivatives significantly block inflammatory pain while having little effect on neurogenic pain, as indicated by the formalin test in mice. Related compounds, like SB-FI-26 , bind fatty acid binding protein 5 (FABP5). This may be related to pain suppression, since FABP5 acts as a transporter of the endocannabinoid anandamide. While certain derivatives of α-truxillic acid can directly activate peroxisome proliferator-activated receptor γ, α-truxillic acid has no such activity.