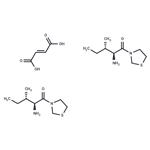
The glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1) are responsible for a majority of nutrient-stimulated insulin secretion. After being released into the circulation, GIP and GLP-1 are inactivated by the circulating enzyme dipeptidyl peptidase IV (DPP IV). DPP IV inhibitors have emerged as a new class of experimental antidiabetic agents. P32/98 is a competitive transition-state substrate analog inhibitor of DPP IV (Ki = 126 nM). At 20 mg/kg/day, it has been used to improve glucose tolerance, insulin sensitivity, and β-cell glucose responsiveness in diabetic rat models.
![Thiazolidine,3-[(2S,3S)-2-aMino-3-Methyl-1-oxopentyl]-, (2E)-2-butenedioate (2:1)](https://www.chemicalbook.com/CAS/20211123/GIF/251572-86-8.gif)