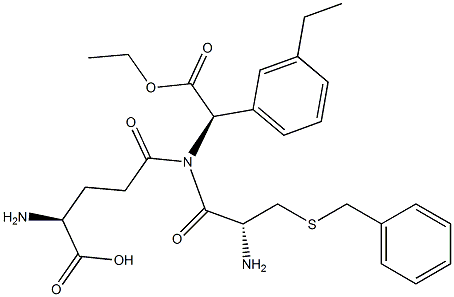
Glutathione S-transferases (GSTs) are ubiquitous multifunctional enzymes that play key roles in cellular detoxification and signal transduction by conjugating target substrates to glutathione. GSTP1-1, the most prevalent GST isozyme in nonhepatic tissues, binds to and inhibits the stress-activated c-Jun N-terminal kinase (JNK), an important regulator of cell proliferation, differentiation, and apoptosis. GSTP1-1 is overexpressed in many cancers and has been linked to drug resistance. Ezatiostat is a tripeptide analog of glutathione that can selectively inhibit GSTP1-1 catalytic activity (Ki = 400 nM) with minimal effect on the related GSTα and -μ families (Kis range from 20-75 μM). After intracellular de-esterification, the active form of ezatiostat, TLK117, binds to and inhibits GSTP1-1, restoring JNK-mediated cellular proliferation and differentiation signaling pathways. Ezatiostat has been shown to stimulate the proliferation of myeloid precursors in preclinical models and is under clinical examination for its potential prevention of myelosuppression in myelodysplastic syndrome.