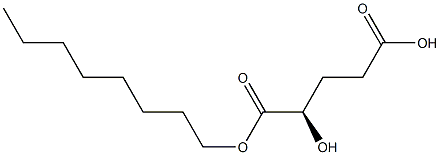
α-Hydroxyglutaric acid (2-HG; ) is normally metabolized to 2-oxoglutarate by D- and L-2-hydroxyglutarate dehydrogenases. Mutations in these enzymes cause 2-hydroxyglutaric aciduria, a neurometabolic disorder. Recent studies have found that mutations in isocitrate dehydrogenase 1 (IDH1) and IDH2, typically associated with certain cancers, can cause these enzymes to convert isocitrate to 2-HG, rather than α-ketoglutarate. 2-HG is structurally similar to α-ketoglutarate and competitively inhibits α-ketoglutarate-dependent dioxygenases, including lysine demethylases and DNA hydroxylases. (2R)-Octyl-α-hydroxyglutarate is a cell-permeable derivative of the D-isomer of 2-HG. It has been used to examine the contribution of D-2HG to the oxidative mitochondrial processes of IDH1-mutated cancer cells.