-
순도시험
(1) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 5.0ppm 이하이어야 한다.
(2) 동 : 이 품목 0.2g을 정밀히 달아 금이 녹을 때까지 왕수를 가하고 가열하여 녹인다. 염화은의 침전물이 생성되는 경우에는 완전히 녹을 때가지 염산을 가한다. 냉각 후, 염산을 가하여 정확히 10mL로 한 액을 시험용액으로 하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 50ppm 이하이어야 한다.
왕수 : 염산 3용량과 질산 1용량을 혼합한다. 사용시에 제조한다.
-
확인시험
(1) 이 품목은 염산, 질산 및 황산에는 녹지 않으나 왕수에는 녹는다.
(2) 이 품목 0.01g에 질산․염산․물의 혼액(1 : 4 : 5) 5mL를 가해주고 가열하여 녹여주고 식힌 다음 염산 2mL를 가하여 수욕상에서 가열 농축한다. 이 조작을 4회 반복하여 질산을 제거한 후 물 20mL를 가한다. 이어서 수산화나트륨시액을 가하여 약산성으로 한 다음 p-디메틸아미노벤지리딘로다닌 [5-(p-dimethylaminobenzylidine)rhodanine]의 에탄올용액(1→3,000) 1mL를 가할 때, 액의 색은 적자색을 나타낸다.
-
정량법
이 품목 약 0.5g을 정밀히 달아 묽은질산(1→2) 20mL를 넣고 50℃이하에서 10분간 가온한 다음 왕수 30mL를 넣어 가열분해시키고 물을 가하여 정확히 100mL로 한다. 이 액 3mL를 정확히 취하여 물을 가하여 200mL로 하고 다시 이 액 5mL를 취하여 물을 가하여 100mL로 한 것을 시험용액으로 한다. 따로 원자흡광광도용 금표준용액(1mL=1,000μg Au) 5mL를 정확히 취한 다음 물을 가하여 50mL로 하고 이 액 2, 4, 6, 8, 10mL를 각각 정확히 취하여 물을 가하여 100mL씩으로 한 액을 각 표준용액으로 한다. 시험용액 및 각 표준용액을 다음의 조작조건에 따라 원자흡광광도법에 따라 시험하고 각 표준용액으로부터 얻어진 검량선으로 부터 검체 중의 금의 함량을 구한다.
조작조건
사용가스 : 가연성가스-아세틸렌 또는 수소
조연성가스-공기
램 프 : 금, 중공음극램프
파 장 : 242.8nm
-
정의
이 품목은 금을 얇은 박으로 만든 것이다.
-
개요
Metallic gold is virtually insoluble, except in aqua regia. Gold exists in three primary forms, elemental, Au (I), and Au (III). As a precious metal, it is resistant to ionization and generally considered biologically benign in its elemental state.

Gold is not permitted for use in foods, drugs or cosmetics as a coloring agent in the U.S. However, surface decoration of foods under conditions which preclude consumption is not considered to be a food use and is, therefore, exempt from regulations concerning food use. The use of gold in the decoration of food is more popular in Europe, where, probably as the result of its limited use, its use has been accepted.
-
화학적 성질
Gold does not have a distinctive odor at room temperature,
but when heated it emits a sweet odor that is detected with
difficulty. Chloroauric acid composed of yellow-orange
crystalline powder has a faint chlorine odor.
-
물리적 성질
Gold is a soft, malleable, ductile, dense metal with a distinctive yellow color. It is almost aheavy as lead, and both can be cut with a knife. One ounce of gold can be beaten and poundedinto a thin sheet that is only a few molecules thick and that will cover over 300 square feetof surface. Although gold is chemically nonreactive, it will react with chlorine and cyanidesolutions and can be dissolved in aqua regia. Its melting point is 1,064.4°C, its boiling pointis 2,808°C, and its density is 19.3 g/cm3 (as compared to lead’s density of 11.35 g/cm3).
-
Isotopes
There are a total of 54 isotopes of gold, only one of which is stable: Au-197,which accounts for the element’s total natural existence on Earth. The remaining 53 isotopesare radioactive, are artificially produced in nuclear reactors or particle accelerators,and have half-lives ranging from a few microseconds to a few seconds to a few hours toa few days.
-
Origin of Name
The name “gold” is Anglo-Saxon as well as from the Sanskrit word javal.
The symbol Au is from the Latin word aurum, which means “shining dawn.”
-
출처
Gold is the 72nd most abundant element and is widely spread around the world, but it is not evenly distributed through the surface of the Earth. It is usually found in a few concentrated regions, sometimes in pure flake and nugget metallic forms. Most of it exists in conjunction with silver ore, quartz (SiO2), and the ores of tellurium, zinc, and copper. About one milligram of gold exists in every ton of seawater (this is about 10 parts of gold per trillion parts of seawater, which amounts to a total of about 79 million tons of gold in solution). No economical method of extracting gold from seawater has been developed to recover this treasury of the sea.
Free metallic gold is found in veins of rocks and in ores of other metals. Alluvial gold (placer deposits) is found in the sand and in the gravel at the bottom of streams where it has been deposited as a result of the movement of water over eons. Most gold is recovered from quartz veins called loads and from ores that are crushed.
-
Characteristics
Gold is not only pleasing to look at but also pleasing to touch, which made it a desirablemetal for human decoration in prehistoric days. It is still the preferred metal for jewelry makingtoday.
Gold is classed as a heavy, noble metal located just below copper and silver in group 11 ofthe periodic table. Gold is a good conductor of electricity as well as an excellent heat reflectorof infrared radiation, which makes it an efficient thin coating on glass in skyscrapers to reflectthe heat of sunlight.
The purity of gold is measured in “carats” (one carat is equal to one part in twenty-four). Thepurest gold is rated at 24 carats, but it is much too soft to be used for jewelry. Good jewelry ismade from 18-carat gold that is 18 parts gold and six parts alloy metal. Thus, an 18-carat goldring is about 75% pure gold and contains about 25% of another metal, such as nickel or copper,to make it harder and more durable. Other alloy metals mixed with gold are silver, platinum, andpalladium—all used to increase gold’s strength and reduce its cost. Some less expensive jewelrycontains 14 or 10 carats of gold (14/24 or 10/24) as well as some other alloy metals.
-
용도
In manufacture of jewelry; in gold plating other metals; as a standard of currency; most frequently alloyed with silver and copper. For use in medicine, see Gold, Radioactive, Colloidal.
Gold has excellent properties as a reflecting mirror. Gold black is utilized as the absorber of light by
depositing on the plane of light incidence of a thermocouple.
-
정의
A transition metal that occurs native.
It is unreactive and is very ductile and malleable.
Gold is used in jewelry, often alloyed
with copper, and in electronics and
colored glass. Pure gold is 24 carat; 9 carat
indicates that 9 parts in 24 consist of gold.
Symbol: Au; m.p. 1064.43°C; b.p.
2807°C; r.d. 19.320 (20°C); p.n. 79; r.a.m.
196.96654.
-
화학 반응
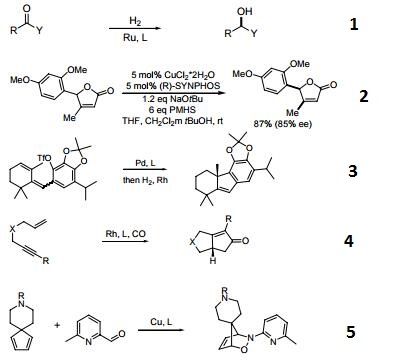
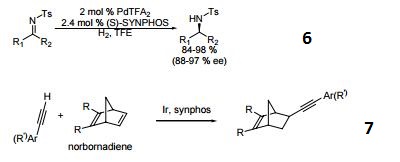
- A chiral diphosphine ligand used in the highly-enantioselective hydrogenation of ketoesters, hydroxyketones, ketophosphonates and succinates.
- A ligand used for the dynamic kinetic resolution of α,β−unsaturated lactones via asymmetric copper-catalyzed conjugate reduction.
- Used in the intramolecular Heck reaction for the synthesis of diterpenoids.
- Used in asymmetric Pauson-Khand reaction.
- Used in asymmetric iminonitroso Diels-Alder reaction.
- Palladium catalyzed asymmetric hydrogenation of N-tosyl ketimines.
- Ligand for asymmetric hydroalkynylation of norbornadienes
-
일반 설명
Gold nanoparticles PEG 5000 biotin terminated.The preparation of biotinylated gold nanoparticles involves two surface modification steps. First, the carboxyl-terminated alkanethiol is attached to the surface of gold nanoparticles via chemisorption in the presence of a stabilizing agent. This step is followed by the reaction of the carboxyl groups with (+)-biotinyl-3,6,9,-trioxaundecanediamine and 2-(2-aminoethoxy)ethanol.
-
위험도
Pure gold, if ingested, can cause skin rash or even a sloughing off of skin. It can also causekidney damage and problems with the formation of white blood cells.
-
Pharmaceutical Applications
Gold has a long-standing tradition in medicine, as it has been used by many nations for thousands of years. From as early as 2500 BC, Arabians, Chinese and Indians used gold compounds for medicinal purposes. In mediaeval times, the elixir aurum potabile , whichwas an alcoholic mixture of herbs with some gold flakes, was sold by medicine men travelling around Europe and this elixir was supposed to cure most diseases. In the nineteenth century, Na[AuCl4] was reported to treat syphilis, whilst others used it to cure alcoholism. On a more serious note, Koch discovered in 1890 the antibacterial properties of gold cyanide. In vitro experiments with the Mycobacterium tuberculosis showed that gold cyanide has the potential as a tuberculosis therapy. Gold compounds were also investigated for the treatment of RA, when it was believed that RA was caused by bacteria, and many other health problems.
-
Safety Profile
Poison by intravenous
route. Questionable carcinogen with
experimental tumorigenic data by
implantation. Can form explosive
compounds with NH3, NH4OH + aqua regia, H2O2. Incompatible with mixtures
containing chlorides, bromides, or iocbdes (if
they can generate nascent halogens), some
oxidizing materials (especially those
containing halogens), alkali cyanides,
thiocyanate solutions, and double cyanides.
See also GOLD COMPOUNDS.
-
Structure and conformation
The space lattice of gold belongs to the cubic system, and its face-centered cubic lattice has a lattice
constant of a=0.40705 nm.