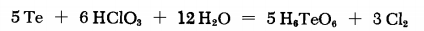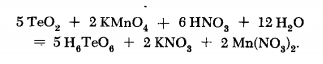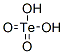
TELLURIC ACID
한글명:TELLURIC ACID
카스 번호13520-55-3
상품명:TELLURIC ACID
CBNumberCB8138828
분자식H6O6Te
포뮬러 무게229.64
MOL 파일13520-55-3.mol
| 위험품 표기 | Xn |
| 위험 카페고리 넘버 | 20 |
| 안전지침서 | 22-36/37/39-38 |
| 유엔번호(UN No.) | UN 3284 6.1/PG 3 |
| WGK 독일 | 3 |
| RTECS 번호 | WY2350000 |
| F 고인화성물질 | 8 |