-
外観
白色~うすい褐色, 粉末
-
性質
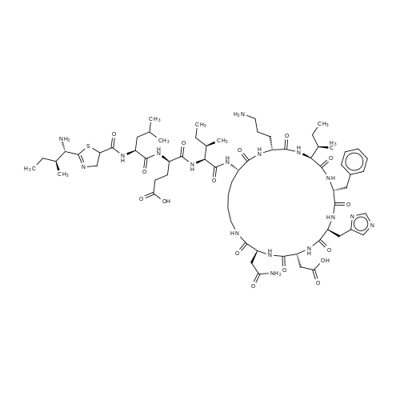
ここでは、バシトラシンAについて説明します。化学式はC66H103N17O16Sで表され、分子量は1,422.71です。CAS番号は1405-87-4で登録されています。化審法官報公示番号は8-474です。
バシトラシンは融点221〜225°Cで、常温で白色粉末の固体です。水に溶けやすく、エタノールにも溶け、エーテルにはほとんど溶けません。
-
溶解性
水に易溶、エタノールに可溶、エーテルにほとんど不溶。
-
解説
バシトラシンA,バシトラシンエー.Bacillus subtilis var Tracyなどの生産する環状ペプチド系抗生物質の主成分.白色または淡灰色の粉末.[α]D25"+5°(0.02 mol L-1 塩酸).λmax 252 nm(水).水,エタノールに可溶.細菌の細胞壁合成系に作用する.グラム陽性菌およびナイセリアの発育を阻止する.感染性口内炎治療薬に用いられる.LD50 190 mg/kg(ラット,静注).
-
用途
細胞同定用ディスク及び培地添加用。
-
用途
ペプチド系抗生物質です。細
菌の細胞壁ペプチドグリカン生合成系を阻害
することで、抗菌作用を示します。ほとん
どはバシトラシン A ですが、バシトラシン
A,B,C,D,E など少なくとも 9 種以上の混合物
です。
-
製法
バシトラシンは、枯草菌であるBacillus licheniformisおよび、一部のBacillus subtilisにより産生されます。
-
説明
Bacitracin is a mixture of similar peptides produced by fermentation of the bacterium Bacillus subtil is. The
A-type component predominates. Its mode of action is to inhibit both peptidoglycan biosynthesis at a late
stage (probably at the dephosphorylation of the phospholipid carrier step) and disruptions of plasma
membrane function.
-
化学的特性
Bacitracin is a white to light tan powder
which is odorless or having a slight odor and very bitter
taste.
-
使用
Bacitracin is a peptide antibiotic effective against gram-postive bacteria. Bacitracin is an inhibitor of peptidoglycan synthesis. Bacitracin disrupts bacterial cell wall synthesis by inhibiting depho
sphorylation of lipid pyrophosphate. Bacitracin also strongly inhibits proline endopeptidase from human muscle.
-
定義
A complex
of cyclic peptide antibiotic of known chemical
structure isolated from the Tracy-I strain of Bacillus
subtilis.
-
適応症
This polypeptide antibiotic, which is produced from Bacillus subtilis,
interferes with bacterial cell wall growth and is bactericidal against many grampositive
organisms such as streptococci, staphylococci, and pneumococci but is
inactive against most gram-negative organisms. All anaerobic cocci, Neisseria, and
the tetanus and diphtheria bacilli are also sensitive to bacitracin.
Resistance is rare,
but some staphylococcal strains are inherently resistant. Hypersensitivity reactions
are uncommon. Bacitracin is stable in petrolatum (but not water-miscible preparations)
and is available as an ointment or as a component of antibiotic mixtures.
Sensitization to bacitracin has been reported more recently, particularly in patients
with leg ulcers.
-
危険性
Poison; moderately toxic; mutagen.
-
使用用途
バシトラシンは、主としてグラム陽性細菌に対し強い抗菌作用を示すため、医薬品として用いられます。細菌の細胞壁の形成を阻害し、DNAを酸化切断することで殺菌作用を示します。作用時間が短く、1日数回の局所投与が必要です。
バシトラシンは筋肉内に投与すると腎毒性を示し、腎不全を引き起こす可能性があります。また、経口摂取でも強い毒性を示すことから、傷口などに局所的に用いるトローチや軟膏などの剤形で利用されます。
-
取り扱い及び保管上の注意
取り扱い時の対策
強酸化剤は、バシトラシンの混触危険物質です。取り扱う時や保管時に、強酸化剤と接触しないよう気を付けてください。
取り扱う際は、必ず保護手袋とゴーグルなどの側板付きの保護メガネ、袖の長い保護衣を着用して皮膚や眼との接触を避け、局所排気装置内で使用してください。
火災の場合
燃焼により、一酸化炭素 (CO) や二酸化炭素 (CO2) 、窒素酸化物 (NOx) 、硫黄酸化物 (SOx) へと分解し、有毒なガスや蒸気を生成するおそれがあります。水噴霧や耐アルコール泡消火剤、粉末消火剤、二酸化炭素、消化砂を用いて消火してください。
眼に入った場合
眼に入った際は、眼を傷つけないように、水でしばらくの間洗浄します。直ちに、医師の診察を受けてください。
吸入した場合
吸入してしまった際は、すぐに新鮮な空気のある場所に移動します。呼吸していない場合は、人工呼吸を施してください。症状が続く場合は、医師の診断を受けてください。
保管する場合
ガラス製の容器に密閉し、2〜10℃の冷蔵庫内で直射日光を避けて保管してください。保管部屋は、施錠します。
参考文献
-
応用例(製薬)
A mixture of peptides produced by Bacillus licheniformis.
Bacitracin A is the major constituent of commercial preparations.
The more stable zinc salt is used in topical formulations.
It has been widely used as a growth promoter in animals, but
has been banned for that purpose in the European Union.
It is highly active against many Gram-positive bacteria
and is mainly used as a component of topical preparations.
Although strains of Staph. aureus are usually susceptible,
they are rather less so than most other Gram-positive bacteria.
Streptococcus pyogenes is so much more susceptible than
other hemolytic streptococci that bacitracin susceptibility is
used as a screening test for identification. Clostridium difficile
and Actinomyces spp. are susceptible, but enterobacteria and
Pseudomonas spp. are resistant. Entamoeba histolytica is inhibited
by 0.6–10 mg/L.
Resistance is uncommon, but has been detected in Staph.
aureus following topical treatment.
It is nephrotoxic and unsuitable for parenteral use. Systemic
toxicity from application to skin or ulcerated areas is rare, but
it may cause allergic reactions and occasional anaphylaxis has
been described. It is found in many ointments and ophthalmic
preparations, usually together with other components,
including polymyxins, neomycin and corticosteroids.
Bacitracin is not absorbed by mouth but oral preparations
have been used for suppression of gut flora, including
C. difficile.
-
臨床応用
Bacitracin is predominantly active against Gram-positive microorganisms, and parenteral use is
limited to IM injection for infants with pneumonia and empyema caused by staphylococci resistant to other
agents. It is rather neuro- and nephrotoxic and, therefore, is used in this manner with caution. Bacitracin
also is widely employed topically to prevent infection in minor cuts, scrapes, and burns.
-
安全性プロファイル
A poison by
intraperitoneal and intravenous routes.
Moderately toxic by ingestion and
subcutaneous routes. Mutation data
reported.
-
職業ばく露
Bacitracin is used as an ingredient in
antibiotic ointments to treat or prevent topical or eye infections.
Commercial Bacitracin is a mixture of at least 9 bacitracins.
Also used as a feed and drinking water additive for
animals; as a food additive for human consumption.
-
輸送方法
UN 3249 Medicine, solid, toxic, n.o.s., Hazard
Class: 6.1; Labels: 6.1—Poisonous materials.
-
純化方法
Bacitracin has been purified by carrier displacement using n-heptanol, n-octanol and n-nonanol as carriers and 50% EtOH in 0.1 N HCl. The pure material gives one spot with RF ~0.5 on paper chromatography using AcOH:n-BuOH:H2O (4:1:5). [Porath Acta Chem Scand 6 1237 1952.] It has also been purified by ion-exchange chromatography. It is a white powder soluble in H2O and EtOH but insoluble in Et2O, CHCl3 and Me2CO. It is stable in acidic solution but unstable in base. It is a strong antibacterial. [Abraham & Bewton Biochem J 47 257 1950, Synthesis: Munekata et al. Bull Chem Soc Jpn 46 3187, 3835 1973, Beilstein 27 III/IV 5746.]
-
不和合性
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions.
Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides.
-
廃棄物の処理
It is inappropriate and possibly
dangerous to the environment to dispose of expired or waste
pharmaceuticals by flushing them down the toilet or discarding
them to the trash. Household quantities of expired or
waste pharmaceuticals may be mixed with wet cat litter or
coffee grounds, double-bagged in plastic, discard in trash.
Larger quantities shall carefully take into consideration
applicable DEA, EPA, and FDA regulations. If possible
return the pharmaceutical to the manufacturer for proper disposal
being careful to properly label and securely package
the material. Alternatively, the waste pharmaceutical shall
be labeled, securely packaged and transported by a state
licensed medical waste contractor to dispose by burial in a
licensed hazardous or toxic waste landfill or incinerator.