-
外観
無色~ほとんど無色, 澄明の液体
-
種類
シクロヘキシルアミンは、研究開発用試薬製品や産業用化学薬品として販売されています。
有機合成原料として使用されることが多く、通常室温で取り扱い可能な試薬製品です。ゴム用薬品、染料、顔料、防錆剤、不凍液などに利用されており、工場などの需要に合わせた大型容量の製品です。
-
性質
シクロヘキシルアミンは、分子量99.17、融点-17.7 ℃、沸点134.5 ℃であり、常温では無色から黄色の液体です。 強い魚臭またはアンモニア類似の臭いを呈します。
密度は0.8627g/mL、酸解離定数pKaは10.64です。 アルコール類、エーテル類、ケトン類、エステル類、脂肪族炭化水素類、芳香族炭化水素類に可溶であり、すなわち一般的な有機溶剤に溶解します。
-
反応性
シクロヘキシルアミンは、 強塩基性であり、酸と激しく反応します。また、加熱や燃焼によって分解し、窒素酸化物などの有毒で腐食性のヒュームを生じます。
強酸化剤と激しく反応して火災の危険を伴うとともに、アルミニウム、銅、亜鉛を侵す物質です。保管の際にはこれらの物質との混触を避けることが必要です。
-
溶解性
水及びアルコール, アセトンに可溶。水、エタノール及びアセトンに極めて溶けやすい。
-
解説
cyclohexanamine.C6H13N(99.18).シクロヘキシルアミン は,アニリンの高温·高圧還元でつくる.アミン臭のある無色の液体.融点-17.7 ℃,沸点134.5 ℃.d204 0.867.n20D 1.459.水,有機溶媒に可溶.引火点32.2 ℃(開放).強塩基性.殺虫剤,腐食防止剤,そのほかの化学合成原料として広く用いられる.工業的には有機溶媒,染料の原料として使われる。皮膚から吸収され,毒性を示す。LD50 710 mg/kg(ラット,経口).森北出版「化学辞典(第2版)
-
用途
ゴム用薬品,清缶剤、染料,顔料,染色助剤、殺虫剤、不凍液、防錆剤
-
用途
有機合成原料、合成甘味剤原料
-
合成
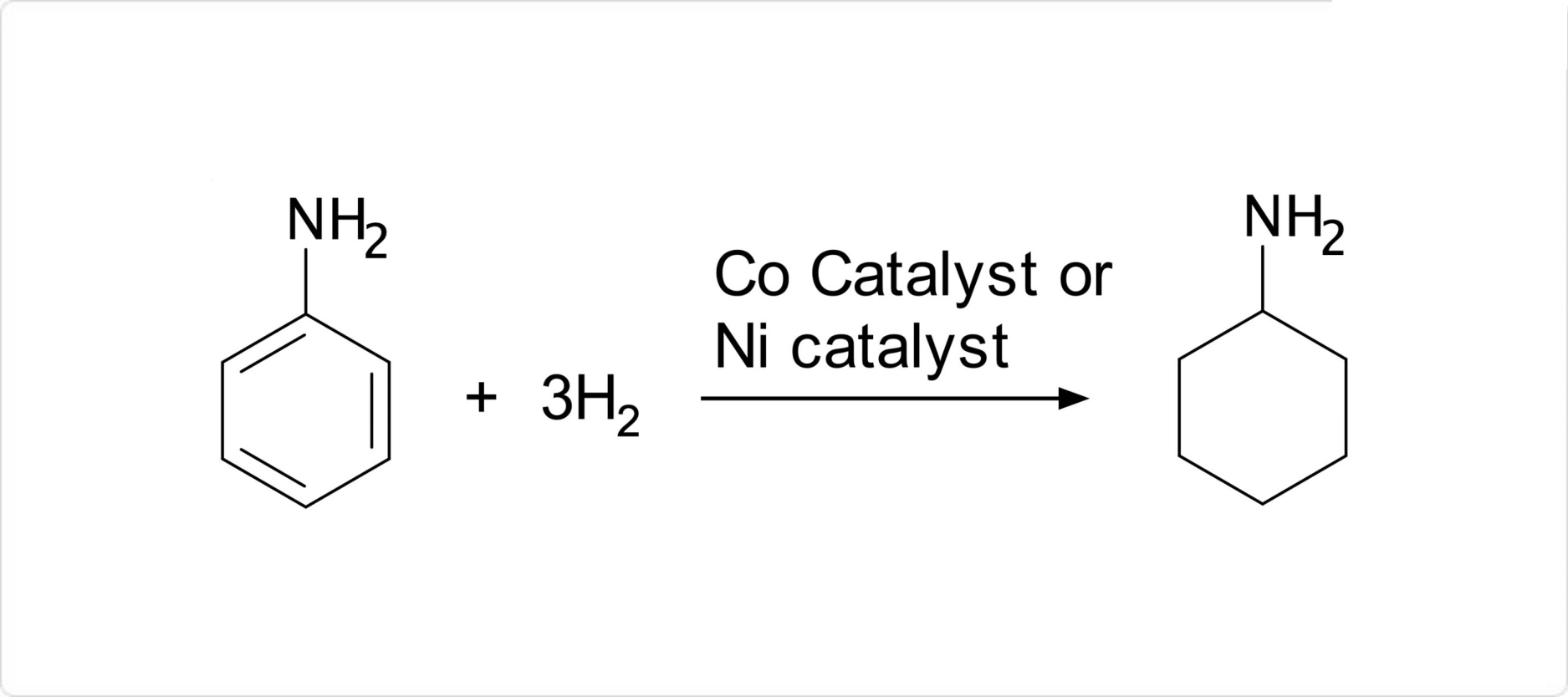
図2. シクロヘキシルアミンの合成
シクロヘキシルアミンの主な合成方法には、やコバルトを用いてを水素化する方法と、シクロヘキサノールをによってアルキル化する方法とがあります。
-
化学的特性
Cyclohexylamine is a colorless to yellow liquid (amines, primary aromatic). It has an unpleasant fishy odor. Flammable. It is infinitely miscible with water and conventional organic solvents. With water it forms an azeotrope that contains 44.2 % cyclohexylamine and boils at 96.4℃. Cyclohexylamine can be volatilized with water vapor. It can absorb carbon dioxide in the air and form a white crystalline carbonate. Aqueous solution is alkaline. 0.01% concentration of aqueous solution pH = 10.5. Its vapor and air to form an explosive mixture.
-
使用
Cyclohexylamine is used in the manufacture of a number of products, including plasticizers, drycleaning soaps, insecticides, and emulsifying agents. It is also used as a corrosion inhibitor and in organic synthesis.
-
定義
ChEBI: Cyclohexylamine is a primary aliphatic amine consisting of cyclohexane carrying an amino substituent. It has a role as a human xenobiotic metabolite and a mouse metabolite. It is a conjugate base of a cyclohexylammonium.
-
調製方法
Cyclohexylamine is produced by the reaction of ammonia and cyclohexanol at
elevated temperature and pressure in the presence of a silica-alumina catalyst
(SRI 1985). It is also prepared by a similar process of catalytic hydrogenation of
aniline at elevated temperature and pressure. Fractionation of the product of this
reaction yields CHA, aniline, and a high-boiling residue containing n-phenylcyclohexylamine
and dicyclohexylamine (Carswell and Morrill 1937). In 1982,
U.S. production was 4.54 metric tons and 739.3 metric tons were imported into the
U.S. (SRI 1985).
-
一般的な説明
Cyclohexylamine appears as a clear colorless to yellow liquid with an odor of ammonia. Flash point 90 °F. Irritates the eyes and respiratory system. Skin contact may cause burns. Less dense than water. Vapors heavier than air. Toxic oxides of nitrogen produced during combustion.
-
空気と水の反応
Highly flammable. Sensitive to air and light. Soluble in water.
-
反応プロフィール
Cyclohexylamine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
-
健康ハザード
Cyclohexylamine is a severe irritant to theeyes, skin, and respiratory passage. Skincontact can produce burns and sensitization;contact of the pure liquid or its concentratedsolutions with the eyes may cause loss ofvision.
The acute oral and dermal toxicity ofcyclohexylamine was moderate in test sub jects. The toxic effects include nausea, vom iting, and degenerative changes in the brain,liver, and kidney. Inhalation of its vaporsat high concentrations may cause a narcoticeffect.
LD50 value, oral (rats): 156 mg/kg
LD50 value, skin (rabbits): 277 mg/klg
Cyclohexylamine may be mutagenic, thetest for which has so far given inconclusiveresults. Administration of this compoundin animals produced a reproductive effect,including embryotoxicity and a reductionin male fertility. Intraperitoneal injectionof the amine in rats caused a dose dependent increase in chromosomal breaks.Roberts and coworkers (1989) studied themetabolism and testicular toxicity of cyclohexylamine (a metabolite of cyclamate)in rats and mice. Chronic dietary administration of 400 mg/kg/day for 13 weeksshowed decrease in organ weigh, histological changes, and testicular atrophy in boththe Wistar and dark agouti DA rats, but to awidely varying extent, while mice exhibitedno evidence of testicular damage.
There is no evidence of carcinogenicityin animals or humans caused by cyclohexy lamine.
-
火災危険
When heated to decomposition, Cyclohexylamine emits highly toxic fumes. Vapor may travel a considerable distance to source of ignition and flash back. Toxic oxides of nitrogen are produced during combustion. Nitric acid; reacts vigorously with oxiding materials. Stable, avoid physical damage, storage with oxidizing material.
-
使用用途
シクロヘキシルアミンの主な使用用途は、清缶剤、防錆材、顔料や染料、ゴム用薬品、染色助剤、殺虫剤、不凍液などです。特に印刷インキ工業では、フラッシング助剤として用いられます。
また、シクロヘキシルアミンは有機合成化学的に有用な物質であるため、医薬品などの各種化合物の中間原料としての用途もあります。シクロヘキシルアミンを原料として合成される主な物質には、スルフェンアミド系加硫促進剤、除草剤であるヘキサジノン、 (油よりも粘度の高い半固体状の) 、人工甘味料であるチクロなどがあります。
医薬品では、粘液溶解薬・鎮痛剤・気管支拡張薬などの薬品の原料として用いられる場合が多いです。
-
安全性と法規制情報
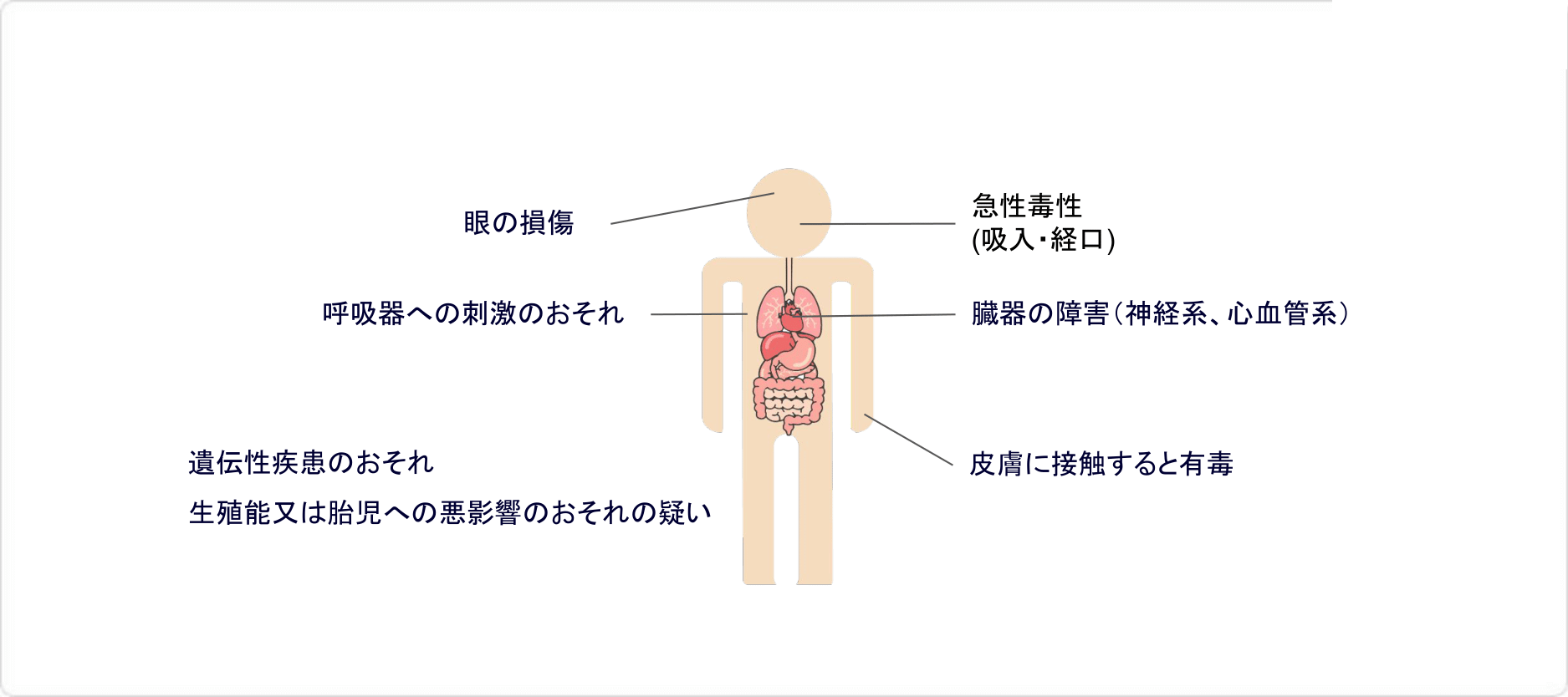
シクロヘキシルアミンの有害性
シクロヘキシルアミンは、 経口摂取・皮膚接触・吸入において有毒な物質です。遺伝性疾患、生殖能又は胎児への悪影響、臓器の障害などの危険性が指摘されており、毒物及び劇物取締法により劇物に指定されています。
また、シクロヘキシルアミンは引火点が26.5 ℃と低い引火性液体であり、26.5℃以上では蒸気/空気の爆発性混合気体を生じることがあるとされている物質です。このため、消防法では「危険物第4類第2石油類」、労働安全衛生法では「危険物・引火性の物、名称等を表示すべき危険有害物」「リスクアセスメントを実施すべき危険有害物」に指定されています。
化学物質排出把握管理促進法 (PRTR法) でも、「第1種指定化学物質」に指定される物質です。法令を遵守して適切に取り扱うことが必要です。
-
工業用途
The primary use of cyclohexylamine is as a corrosion inhibitor in boiler water
treatment and in oil field applications (HSDB 1989). It is also a chemical
intermediate for rubber processing chemicals, dyes (acid blue 62, former use),
cyclamate artificial sweeteners and herbicides and a processing agent for nylon
fiber production (SRI 1985). Windholz et al (1983) reports its use in the manufacture
of insecticides, plasticizers, emulsifying agents, dry-cleaning soaps, and acid
gas absorbents.
-
安全性プロファイル
A poison by ingestion,
skin contact, and intraperitoneal routes.
Experimental teratogenic and reproductive
effects. A severe human skin irritant. Can
cause dermatitis and convulsions. Human
mutation data reported. Questionable
carcinogen. Flammable liquid. Dangerous
fire hazard when exposed to heat, flame, or
oxidizers. To fight fire, use alcohol foam,
CO2, dry chemical. When heated to
decomposition it emits toxic fumes of NOx.
-
合成
Prepared by catalytic hydrogenation of aniline at elevated temp and pressures. Fractionation of crude reaction product yields cyclohexylamine, unchanged aniline, and high-boiling residue containing n-phenylcyclohexylamine (cyclohexylaniline) and dicyclohexylamine.
-
職業ばく露
CHA is used in making dyes, chemi-
cals, dry cleaning chemicals; insecticides, plasticizers, rub-
ber chemicals; and as a chemical intermediate in the
production of cyclamate sweeteners. Used in water treat-
ment and as a boiler feedwater additive. It is also used in
rubber production to retard degradation.
-
発がん性
According to the International Agency for Research of Cancer (IARC) working group, there is no evidence that cyclohexylamine is teratogenic or carcinogenic.
-
貯蔵
Cyclohexylamine can be stored and shipped in iron tanks. Nonferrous metals, particularly copper-containing materials, are attacked and are therefore unsuitable. The amine discolors on contact with air and therefore must be kept under nitrogen.
-
輸送方法
UN2357 Cyclohexylamine, Hazard class: 8;
Labels: 8-Corrosive material, 3-Flammable liquid.
-
純化方法
Dry the amine with CaCl2 or LiAlH4, then distil it from BaO, KOH or Na, under N2. Also purify it by conversion to the hydrochloride (which is crystallised several times from water), then liberation of the amine with alkali and fractional distillation under N2. The hydrochloride has m 205-207o (dioxane/EtOH). [Lycan et al. Org Synth Coll Vol II 319 1943, Beilstein 12 III 10, 12 IV 8.]
-
不和合性
May form explosive mixture with air.
Cyclohexylamine is a strong base: it reacts violently with
acid. Contact with strong oxidizers may cause fire and
explosion hazard. Incompatible with organic anhydrides;
isocyanates, vinyl acetate; acrylates, substituted allyls;
alkylene oxides; epichlorohydrin, ketones, aldehydes, alco-
hols, glycols, phenols, cresols, caprolactum solution; lead.
Corrosive to copper alloys, zinc, or galvanized steel.
-
廃棄物の処理
Incineration; incinerator
equipped with a scrubber or thermal unit to reduce nitrogen
oxides emissions.