-
種類
三フッ化ホウ素は、産業用高圧ガスとして販売されている他、メタノール錯塩 (メタノール溶液) としては研究開発用試薬製品としても販売されています。
産業用高圧ガス製品は、半導体製造、医薬中間体合成触媒、重合触媒等の用途を想定して販売され、1kg、30kgスチール製ボンベや300kgカードルで提供されます。
研究開発用試薬製品は、メタノール錯塩 BF3・CH3OHとして販売されています。5mL、100mL、250mL、500mL、25g、100g、400gなど、実験室で取り扱いやすい容量での提供です。通常室温で保管可能な試薬製品として取り扱われます。通常の試薬グレードの他、ガスクロマトグラフ用など分析用のグレードの製品も存在するため、用途に合わせて選択することが必要です。
-
性質
三フッ化ホウ素は、分子量67.82、融点-126.8℃、沸点-100.3℃であり、常温では無色の気体です。なお、二水和物の分子量は103.837であり、常温では無色の液体です。
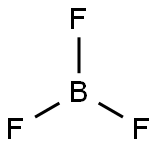
密度は0.00276g/mL (二水和物は1.64g/mL) であり、水に溶解する他、プロパン、ペンタン、ケロセン、ナフサ、クロロホルム、ベンゼン、ニトロベンゼン、ジクロロベンゼンなどの各種有機溶媒に溶けます。分子構造はホウ素原子に3つのフッ素原子が結合し、正三角形状に並んだ構造です。
-
反応性
三フッ化ホウ素は、腐食性がある物質です。ステンレス鋼やモネル、ハステロイなどは、水蒸気の存在下で腐食されます。ポリアミドとの反応性もありますが、テフロンやポリプロピレンなどは腐食されません。
ホウ素は電子不足であり、化学反応においてはルイス酸として働く物質です。例えば、フッ化物と反応してテトラフルオロホウ酸塩を生成します。また、他のハロゲン化ホウ素とは異なって加水分解を受けます。加水分解による生成物は、ホウ酸とホウフッ化水素酸です。
-
用途
触媒、半導体製造用(ドーピング用)、光ファイバー製造用
-
合成
三フッ化ホウ素は、工業的には酸化ホウ素とフッ化水素の反応で合成されます。実験室スケールでは、テトラフルオロホウ酸のジアゾニウム塩の分解や、テトラフルオロホウ酸ナトリウムとフッ化水素酸と硫酸を用いた反応などにより合成されています。
-
化学的特性
Boron trifluoride is a nonflammable, colorless gas with an acrid suffocating odor. It forms thick acidic fumes in moist air. Dry boron trifluoride is used with mild steel, copper, copper-zinc and copper-silicon alloys, and nickel. Moist gas is corrosive to most metallic materials and some plastics. Therefore, Kel-F and Teflon are the preferred gasketing materials. Mercury containing manometers should not be used because boron trifluoride is soluble in mercury. It decomposes in hot water yielding hydrogen fluoride, Shipped as a nonliquefied compressed gas.
-
物理的性質
Colorless gas; pungent suffocating odor; density 2.975 g/L; fumes in moist air; liquefies at -101°C; solidifies at -126.8°; vapor pressure at -128°C is 57.8 torr; critical temperature -12.2°C; critical pressure 49.15 atm; critical volume 115 cm3/mol; soluble in water with partial hydrolysis; solubility in water at 0°C 332 g/100g; also soluble in benzene, toluene, hexane, chloroform and methylene chloride; soluble in anhydrous concentrated sulfuric acid.
-
使用
Boron trifluoride is used as a catalyst for polymerizations, alkylations, and condensation reactions; To protect molten magnesium and its alloys from oxidation; as a gas flux for internal soldering or brazing; in ionization chambers for the detection of weak neutrons; and as a source of B10 isotope. By far the largest application of boron trifluoride is in catalysis with and without promoting agents.
-
使用
Boron trifluoride is the
most widely used boron halide. Most applications take advantage of its strong Lewis acidity.
The most important uses are in organic syntheses.
Boron trifluoride is commonly used as a catalyst
for Friedel-Crafts alkylation reactions. It also is
used to catalyze the cleavage of ethers to alcohols, to catalyze esterification reactions, and in
the nitration and sulfonation of aromatic compounds. Many olefin polymerization reactions
use BF3 as an initiator, in conjunction with a
proton donor, such as water. Also BF3 is used to
catalyze the isomerization of alkenes and alkanes
and in petroleum cracking and desulfurization.
Amine complexes of BF3 are used as epoxy
curing agents.
-
製造方法
Boron trifluoride is prepared by treating borax with hydrofluoric acid; or boric acid with ammonium bifluoride. The complex intermediate product is then treated with cold fuming sulfuric acid.
-
一般的な説明
Boron trifluoride is a colorless gas with a pungent odor. Boron trifluoride is toxic by inhalation. Boron trifluoride is soluble in water and slowly hydrolyzed by cold water to give off hydrofluoric acid, a corrosive material. Its vapors are heavier than air. Prolonged exposure of the containers to fire or heat may result in their violent rupturing and rocketing.
-
空気と水の反応
Fumes in air. Soluble in water and slowly hydrolyzed by cold water to give hydrofluoric acid. Reacts more rapidly with hot water.
-
反応プロフィール
Boron trifluoride is a colorless, strongly irritating, toxic gas. Upon contact with water, steam or when heated to decomposition, Boron trifluoride will produce toxic fluoride fumes. Incompatible with alkyl nitrates, calcium oxide. Reaction with alkali metals or alkaline earth metals (except magnesium) will cause incandescence [Bretherick, 5th ed., 1995, p. 65].
-
危険性
Toxic by inhalation, corrosive to skin and
tissue. Lower respiratory tract irritant, and pneu-
monitis.
-
健康ハザード
Boron trifluoride (and organic complexes such as BF3-etherate) are extremel corrosive substances that are destructive to all tissues of the body. Upon contact with moisture in the skin and other tissues, these compounds react to form hydrofluoric acid and fluoroboric acid, which cause severe burns. Boron trifluoride gas is extremely irritating to the skin, eyes, and mucous membranes. Inhalation of boron trifluoride can cause severe irritation and burning of the respiratory tract, difficult breathing, and possibly respiratory failure and death. Exposure of the eyes to BF can cause severe burns and blindness. This compound is not considered to have adequate warning properties. Boron trifluoride has not been found to be carcinogenic or to show reproductive or developmental toxicity in humans. Chronic exposure to boron trifluoride gas can cause respiratory irritation and damage.
-
火災危険
When heated to decomposition or upon contact with water or steam, Boron trifluoride will produce toxic and corrosive fumes of fluorine containing compounds. Decomposes upon heating or on contact with moist air, forming toxic and corrosive fumes of boric acid and hydrofluoric acid. Reacts with alkalis and fumes in moist air, producing particulates which reduce visibility. Reacts with alkali metals, alkaline earth metals (except magnesium), alkyl nitrates, and calcium oxide. Boron trifluoride hydrolyzes in moist air to form boric acid, hydrofluoric acid, and fluoboric acid.
-
燃焼性と爆発性
Boron trifluoride gas is noncombustible. Water should not be used to extinguish any
fire in which boron trifluoride is present. Dry chemical powder should be used for
fires involving organic complexes of boron trifluoride.
-
使用用途
三フッ化ホウ素の主な使用用途は、触媒 (ルイス酸触媒など) 、半導体製造用途 (ドーピング用) 、重合開始剤、光ファイバー製造用途などです。
1. 触媒
三フッ化ホウ素はアンモニアやジエチルエーテルなどのルイス塩基と容易に複合体を形成し、ルイス酸触媒として有機合成分野で用いられる物質です。触媒する反応には、例えば異性化やアルキル化、エステル化、縮合反応などの反応があります。
特に、ジエチルエーテルとの錯体は蒸留が可能なほど安定であり、市販もされている物質です。
2. 半導体製造
三フッ化ホウ素は、半導体製造の分野では、イオン注入におけるドーパントや、エピタキシャル成長されたシリコンがP型半導体となる際のドーパントとして利用されています。ドーパントとは、半導体に混入させる不純物です。
三フッ化ホウ素は、電子機器や光ファイバーなどに用いられる半導体を製造する際にドーピング源として利用されています。
3. 重合開始剤
三フッ化ホウ素は、重合開始材としても利用されています。重合開始剤としての三フッ化ホウ素は、ビニルフェノールやその誘導体の重合をする際に、限定した分子量分布で重合を行うことができる重合開始材です。
また、三フッ化ホウ素を用いることでリビング重合性を有したカチオン重合を実現することができます。
-
分子構造
三フッ化ホウ素の分子構造は正三角形状であり、共有結合は強く分極しています。ただし、分子自体は非極性です。これはホウ素原子がsp2軌道を取っており、分子が3回対称であるためです。
-
材料の用途
Dry boron trifluoride does not react with the
common metals of construction, but If moisture
is present the acidic hydrates formed (BF3·H2O
and BF3·2H2O) can corrode many common metals
rapidly. Consequently, lines, pressure regulators,
and valves in boron trifluoride service
must be well protected from the entrance of
moist air between periods of use. Cast iron must
not be used because active fluorides attack its
structure. If steel piping is used for boron
trifluoride, forged-steel fittings must be used
instead of cast-iron fittings. Stainless steel, Monel,
nickel, and Hastelloy C are good materials
of construction.
Among materials suitable for gaskets are
Teflon, Kel F, and other appropriate fluorocarbon
or chlorofluorocarbon plastics. Most plastics
become embrittled in boron trifluoride
service. The use of polyvinyl chloride should be
avoided.
-
職業ばく露
Boron trifluoride is a highly reactive
chemical used primarily as a catalyst in chemical synthesis.
It is stored and transported as a gas, but can be reacted
with a variety of materials to form both liquid and solid
compounds. The magnesium industry utilizes the fireretardant
and antioxidant properties of boron trifluoride
in casing and heat treating. Nuclear applications of boron
trifluoride include neutron detector instruments; boron-10
enrichment and the production of neutroabsorbing salts for
molten-salt breeder reactors.
-
概要
三フッ化ホウ素 (英: Boron trifluoride) とは、ホウ素のフッ化物の1種で、化学式BF3で表される無機化合物です。
無水和物の他、一般的には二水和物があります。それぞれCAS登録番号は、7637-07-2と13319-75-0です。
粘膜に対して刺激性を持つ気体であるため、取り扱いには注意が必要です。ジエチルエーテルと形成する錯体は液体であり、しばしばルイス酸として用いられます。
-
貯蔵
All work with boron trifluoride should be conducted in a
fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves
should be worn to prevent eye and skin contact. Cylinders of boron trifluoride should be
stored in locations appropriate for compressed gas storage and separated from alkali metals,
alkaline earth metals, and other incompatible substances. Solutions of boron trifluoride should
be stored in tightly sealed containers under an inert atmosphere in secondary containers.
-
輸送方法
UN1008 Boron trifluoride, Hazard class: 2.3;
Labels: 2.3—Poisonous gas, 8—Corrosive material,
Inhalation Hazard Zone B. Cylinders must be transported
in a secure upright position, in a well-ventilated truck.
Protect cylinder and labels from physical damage. The
owner of the compressed gas cylinder is the only entity
allowed by federal law (49CFR) to transport and refill
them. It is a violation of transportation regulations
to refill compressed gas cylinders without the express
written permission of the owner.
-
純化方法
The usual impurities-bromine, BF5, HF and non-volatile fluorides-are readily separated by distillation. Brown and Johannesen [J Am Chem Soc 72 2934 1950] passed BF3 into benzonitrile at 0o until the latter was saturated. Evacuation to 10-5mm then removed all traces of SiF4 and other gaseous impurities. [A small amount of the BF3-benzonitrile addition compound sublimes and is collected in a U-tube cooled to -80o]. The pressure is raised to 20mm by admitting dry air, and the flask containing the BF3 addition compound is warmed with hot water. The BF3 that evolves is passed through a -80o trap (to condense any benzonitrile) into a tube cooled in liquid air. The addition compound with anisole can also be used. BF3 can be dried by passing it through H2SO4 saturated with boric oxide. It fumes in moist air. [It is commercially available as a 1.3M solution in MeOH or PrOH.] [Booth & Wilson Inorg Synth I 21 1939, Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 219-222 1963.] TOXIC.
-
不和合性
Boron trifluoride reacts with polymerized
unsaturated compounds. Decomposes on contact with
water, moist air, and other forms of moisture, forming toxic
and corrosive hydrogen fluoride, fluoroboric acid, and boric
acid. Reacts violently with alkali and alkaline earth metals
(except magnesium); metals, such as sodium, potassium,
and calcium oxide, and with alkyl nitrates. Attacks many
metals in presence of water.
-
廃棄物の処理
Return refillable compressed
gas cylinders to supplier. The owner of the compressed gas
cylinder is the only entity allowed by federal law (49CFR)
to transport and refill them. Chemical reaction with water
to form boric acid, and fluoroboric acid. The fluoroboric
acid is reacted with limestone, forming boric acid and calcium
fluoride. The boric acid may be discharged into a sanitary
sewer system while the calcium fluoride may be
recovered or landfilled. Protect cylinder and labels from
physical damage.
-
予防処置
Exposures to boron trifl uoride in occupational work areas cause irritating effects,
painful burns, lesions, and loss of vision. Workers with potential exposure to boron
trifl uoride should not wear contact lenses. Prompt medical attention is mandatory
in all cases of overexposure to boron trifl uoride and the rescue personnel should be
equipped with proper protectives. Occupational workers should handle/use boron trifl uoride only in well-ventilated areas. The valve protection caps must remain in place.
Workers should not drag, slide, or roll the cylinders, and use a suitable hand truck for
cylinder movement. Compressed gas cylinders shall not be refi lled without the express
written permission of the owner. Boron trifl uoride is listed as an extremely hazardous
substance (EHS).
The cylinder should not be heated by any means to increase the discharge rate of the
product from the cylinder. The cylinder of boron trifl uoride should be kept stored in a cool,
dry, well-ventilated area of non-combustible construction away from heavily traffi cked
areas and emergency exits