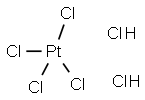-
種類
塩化白金酸は、前述の通り、ヘキサクロリド白金(IV)酸 (H2[PtCl6]) とテトラクロリド白金(II)酸 (H2[PtCl4]) の2種類が存在しますが、主に販売されているのはヘキサクロリド白金(IV)酸であり、テトラクロリド白金(II)酸はカリウム塩などの状態で販売されています。
1. ヘキサクロリド白金(IV)酸
ヘキサクロリド白金(IV)酸は、主に水和物の状態で研究開発用試薬や、貴金属薬品として販売されています。研究開発用試薬製品は、主に六水和物 (H2[PtCl6]・6H2O) として販売されており、1g、5g、25gなどの小容量で提供されています。通常、冷蔵で保管されることの多い試薬製品です。
また、貴金属薬品としては、白金試薬合成用材料や触媒製造、メッキ材料として、n水和物の他、塩酸溶液製品も販売されています。貴金属薬品であることから、工業用薬品としても100gなどの比較的小容量で提供されている物質です。
2. テトラクロリド白金(II)酸
テトラクロリド白金(II)酸は、基本的には一般販売されておらず、ナトリウム塩の水和物 (テトラクロロ白金酸(II)ナトリウム水和物: Na2[PtCl4]・nH2O) や、カリウム塩(テトラクロロ白金(II)酸カリウム: K2[PtCl4]) などとして販売されています。
試薬製品として販売されていることが一般的であり、1g、5g、10gなどの小容量で提供されている物質です。通常、室温で保管可能な試薬製品として取り扱われています。
-
定義
本品は、次の化学式で表される無機化合物である。
-
性質
ヘキサクロリド白金(IV)酸は (英: Hexachloroplatinic(IV) acid) 六水和物として取り扱われることの多い物質です。
この六水和物は、分子量517.891、融点60℃であり、常温では赤褐色の固体です。強い潮解性を示し、水に極めてよく溶けます。水溶液は、強酸を示し、また、やにも溶けます。密度は2.431g/mLであり、CAS登録番号は16941-12-1です。
-
解説
塩化白金酸,白金塩化水素酸ともいう。酸化数+IIおよび+IVの白金を含む酸とその塩が知られている。[テトラクロロ白金(II)酸] 化学式H2[PtCl4]。H2[PtCl6]水溶液を還元すると赤色溶液として得られる。この溶液に金属の水酸化物を溶かすと塩MI2[PtCl4](MIは1価陽イオン)が得られる。塩は赤色結晶。アルカリ塩などは水に易溶。[PtCl4]2-は平面正方形型構造をもつ。カリウム塩K2[PtCl4](比重3.382(25℃)),セシウム塩Cs2[PtCl4],バリウム塩Ba[PtCl4]・3H2O(比重2.868)などがある。
-
化粧品の成分用途
表面改質剤
-
化学的特性
Orange/Red Crystals
-
使用
Chloroplantinic acid (H2PtCl6) is one of the most commercially important compounds of
platinum. Its many uses include etching on zinc, making indelible ink, plating, and coloring
in fine porcelains and use in photography, in mirrors, and as a catalyst.
-
定義
chloroplatinic acid: A reddish crystallinecompound, H2PtCl6, made bydissolving platinum in aqua regia.
-
一般的な説明
Chloroplatinic acid, is a reddish-brown solid. Chloroplatinic acid is soluble in water and will yield a mildly acidic solution. Chloroplatinic acid may cause illness from inhalation of the dust and Chloroplatinic acid is irritating to skin and eyes. When heated to high temperatures Chloroplatinic acid may decompose to toxic chloride fumes. Chloroplatinic acid may burn, but may be difficult to ignite. Chloroplatinic acid is used for manufacturing indelible ink and in electroplating processes.
-
空気と水の反応
Soluble in water.
-
反応プロフィール
Oxidizing acids are generally soluble in water with the release of hydrogen ions. The resulting solutions have pH's of less than 7.0. Materials in this group react with chemical bases (for example: amines and inorganic hydroxides) to form salts. These neutralization reactions occur as the base accepts hydrogen ions that the acid donates. Neutralizations can generate dangerously large amounts of heat in small spaces. The dissolution of acids in water or the dilution of their concentrated solutions with water may generate significant heat. The addition of water acids often generates sufficient heat in the small region of mixing to boil some of the water explosively. The resulting "bumping" spatters acid widely. These materials have significant ability as oxidizing agents. but that ability varies (for example, from high for nitric acid to low for sulfuric acid and most sulfonic acids). They can react with active metals, including iron and aluminum, and also many less active metals, to dissolve the metal and liberate hydrogen and/or toxic gases. Like other acids, materials in this group can initiate polymerization in certain classes of organic compounds. Their reactions with cyanide salts and compounds release gaseous hydrogen cyanide. Flammable and/or toxic gases are also often generated by their reactions with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and weak or strong reducing agents. Additional gas-generating reactions occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and even carbonates: the carbon dioxide gas from the last is nontoxic but the heat and spattering from the reaction can be troublesome. Acids often catalyze (increase the rate) of chemical reactions.
-
健康ハザード
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
-
火災危険
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
-
使用用途
塩化白金酸の主な用途は、白金化合物合成、触媒の製造の出発物質、分析における白金イオンの供給源、分析試薬などです。塩化白金酸の濃溶液に、などを浸し、焼いて分解させることにより、白金石綿という酸化触媒を得ることができます。白金石綿は、水素や酸素の生成などに使われています。
また、ヘキサクロリド白金酸水溶液に塩化物水溶液を加え、濃縮することでヘキサクロリド白金酸塩が析出します。ヘキサクロリド白金酸塩は、さまざまな場面で使用されています。例えば、ヘキサクロリド白金酸アンモニウムは、白金メッキに使われる物質です。
-
塩化白金酸のその他情報
塩化白金酸の合成
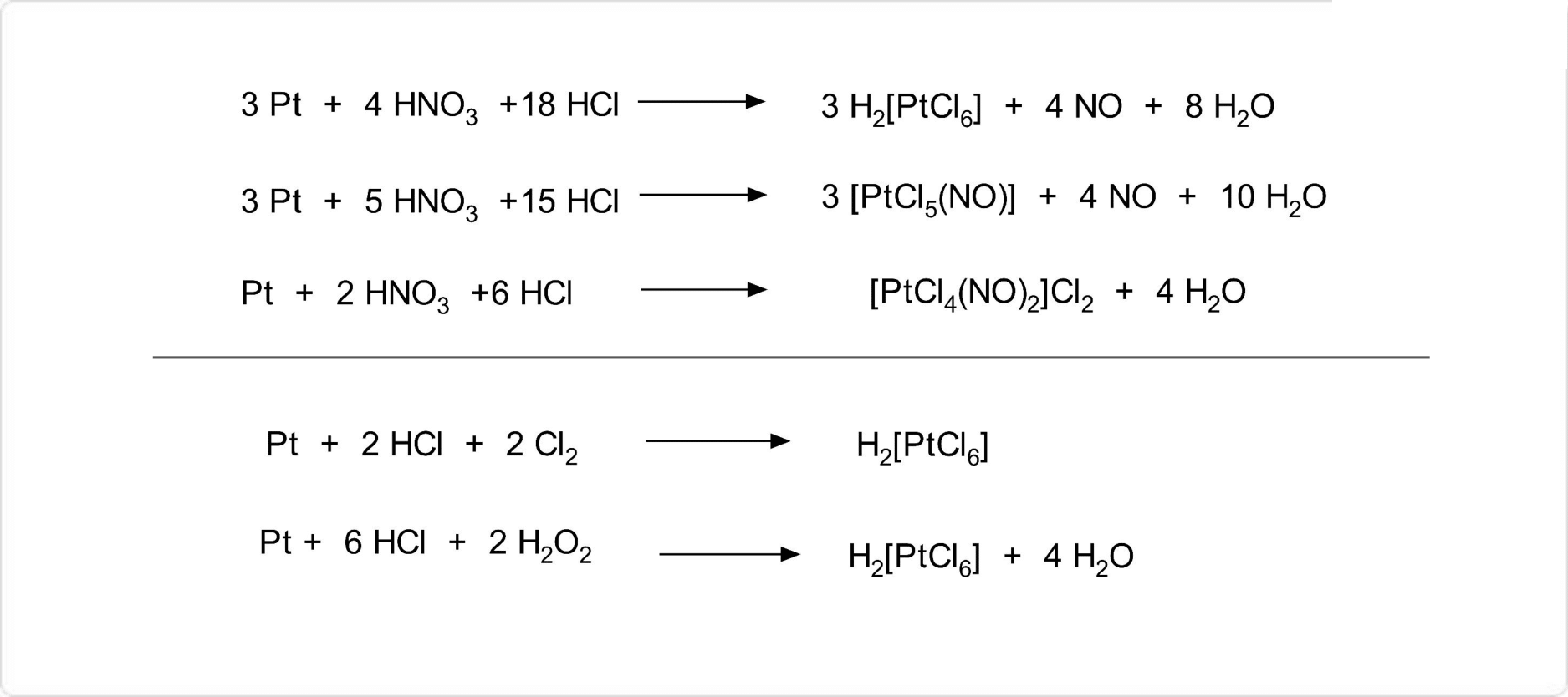
図3. ヘキサクロリド白金(IV)酸の合成
ヘキサクロリド白金(IV)酸の合成方法の一つは、白金を熱王水に溶かすことです。この方法ではニトロシル (NO+配位子) が生じやすいため、濃塩酸を加え蒸発乾固によって濃縮することを繰り返します。ただし、ニトロシルを完全に除去することは難しいため、次の2つの方法によって合成する方が純粋なヘキサクロリド白金(IV)酸を得やすいとされています。
- 白金粉末を温めた濃塩酸に懸濁させ、撹拌しながら塩素ガスを通じる
- 白金粉末を温めた濃塩酸に懸濁させ、過酸化水素水を滴下して発生する塩素により酸化溶解させる
参考文献
-
安全性プロファイル
Poison by intravenous
and intraperitoneal routes. Mutation data
reported. See PLATINUM COMPOUNDS
and CHLORIDES. Incompatible with BrF3.
When heated to decomposition it emits
toxic fumes of Cl-.
-
職業ばく露
Chloroplatinic acid has many uses, among them are platinum plating, photography, and catalysis.
-
輸送方法
UN2507 Chloroplatinic acid, solid, Hazard class: 8; Labels: 8-Corrosive material.
-
純化方法
If it is to be purified, or regenerated from Pt recovered from catalytic hydrogenations, it should be dissolved in aqua regia followed by evaporation to dryness and dissolution in the minimum volume of H2O. Then the aqueous solution is treated with saturated ammonium chloride until all the ammonium hexachloroplatinate separates. The (NH4)2PtCl6 is filtered off and dried at 100o. Igniting this salt gives Pt sponge; dissolve the Pt sponge in aqua regia, boil to dryness, dissolve the residue in concentrated HCl, boil to dryness again and repeat the process. Protect it from light. [Hickers J Am Chem Soc 43 1268 1921, Adams et al. Org Synth Coll Vol I 463, 466 1941, Bruce J Am Chem Soc 58 687 1936.]
-
不和合性
Oxidizing acids are generally soluble in water with the release of hydrogen ions. The resulting solutions have pH’s of <7.0. Materials in this group react with chemical bases (e.g., amines and inorganic hydroxides) to form salts. These neutralization reactions occur as the base accepts hydrogen ions that the acid donates. Neutralizations can generate dangerously large amounts of heat in small spaces. The dissolution of acids in water or the dilution of their concentrated solutions with water may generate significant heat. The addition of water acids often generates sufficient heat in the small region of mixing to boil some of the water explosively. The resulting “bumping” spatters acid widely. These materials have significant ability as oxidizing agents. but that ability varies (e.g., from high for nitric acid to low for sulfuric acid and most sulfonic acids). They can react with active metals, including iron and aluminum, and also many less active metals, to dissolve the metal and liberate hydrogen and/or toxic gases. Like other acids, materials in this group can initiate polymerization in certain classes of organic compounds. Their reactions with cyanide salts and compounds release gaseous hydrogen cyanide. Flammable and/or toxic gases are also often generated by their reactions with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and weak or strong reducing agents. Additional gas-generating reactions occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and even carbonates: the carbon dioxide gas from the last is nontoxic but the heat and spattering from the reaction can be troublesome. Acids often catalyze (increase the rate) of chemical reactions.