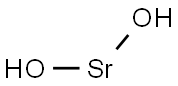ChemicalBook > CAS DataBase List > Strontium hydroxide
Strontium hydroxide
Strontium hydroxide
- CAS No.18480-07-4
- Chemical Name:Strontium hydroxide
- CBNumber:CB9226504
- Molecular Formula:H2O2Sr
- Formula Weight:121.63
- MOL File:18480-07-4.mol
Strontium hydroxide Property
- Melting point 535°C
- Boiling point 710 °C (decomposes)
- Density 3.625
- vapor pressure 0Pa at 20℃
- refractive index 1.650
- form colorless orthorhombic crystals
- color colorless orthorhombic crystals, crystalline; hygroscopic
- Odor Odorless
- PH 11.27(1 mM solution);12.22(10 mM solution);13.09(100 mM solution)
- Water Solubility g/100g solution in H2O: 0.90 (0°C), 2.16 ± 0.41 (25°C), 47.71 (100°C); solid phase, Sr(OH)2 · 8H2O [KRU93]
- Merck 13,8924
- Stability Stable.
- CAS DataBase Reference 18480-07-4(CAS DataBase Reference)
- EWG's Food Scores 1-3
- FDA UNII EPK818UET5
- NIST Chemistry Reference Strontium hydroxide(18480-07-4)
- EPA Substance Registry System Strontium hydroxide (Sr(OH)2) (18480-07-4)
- UNSPSC Code 12352302
- NACRES NA.23
Safety
- Hazard Codes :C
- Risk Statements :34
- Safety Statements :26-27-36/37/39-45
- RIDADR :UN 3262 8/PG 2
- WGK Germany :3
- RTECS :WK9100000
- HazardClass :8
- PackingGroup :III
- HS Code :28164000
-
NFPA 704:
0 2 0
-
Symbol(GHS)

- Signal wordDanger
- Hazard statements H314
- Precautionary statements P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338-P363
Strontium hydroxide Price
More Price(1)
- Brand: Sigma-Aldrich(India)
- Product number: 433608
- Product name : Strontium hydroxide
- Purity: 94%
- Packaging: 25G
- Price: ₹3041.83
- Updated: 2022/06/14
- Buy: Buy
Strontium hydroxide Chemical Properties,Usage,Production
- Uses Strontium hydroxide is used in extracting sugar from beet sugar molasses and in making lubricant soaps and greases.
- Toxicity Dry compound or aqueous solution is corrosive. Contact with skin or eyes can cause irritation.
- Description Strontium Hydroxide occurs as an anhydrate whose prismatic, colorless crystals are deliquescent. A monohydrate and octahydrate are also known.Strontium hydroxide is slightly soluble in water but, surprisingly, the octahydrate is much more soluble. It is the latter that is supplied commercially for most applications.When the octahydrate is heated, seven waters of hydration are lost to form the monohydrate and finally the oxide.
- Chemical Properties colourless deliquescent crystals
-
Physical properties
White deliquescent crystal; density 3.625 g/cm3; melts at 375°C in hydrogen atmosphere; converts to oxide at 710°C; slightly soluble in water at 0°C, 0.41 g/100 mL, soluble in boiling water at 100°C, 21.83 g/100 mL; soluble in acids and ammonium chloride solution.
The octahydrate consists of colorless, tetragonal, deliquescent crystals; density 1.90 g/cm3; loses all its water molecules at 100°C; sparingly soluble in water at low temperatures, 0.90 g/100 mL at 0°C; soluble in boiling water, 47.7 g/100 mL at 100°C; aqueous solution highly alkaline; soluble in acids and in ammonium chloride solution; insoluble in acetone. - Uses Extraction of sugar from beet sugar molasses, lubricant soaps and greases, stabilizer for plastics, glass, adhesives.
- Uses Strontium hydroxide is used chiefly in the refining of beet sugar and as a stabilizer in plastic. It may be used as a source of strontium ions when the Cl- ion is undesirable. Strontium hydroxide absorbs carbon dioxide from the air to form strontium carbonate. This salt is available commercially in sizable quantities.
- Uses Strontium hydroxide [Sr(OH)2] is used to extract sugar from sugar beet molasses. It is also used in the manufacture of soaps, adhesives, plastics, glass, and lubricants that can be used in very high or low temperature environments.
- Definition ChEBI: Strontium dihydroxide is a strontium hydroxide.
-
Preparation
Preparation Strontium hydroxide is prepared by treating strontium oxide with water: SrO + H2O → Sr(OH)2
Alternatively, Sr(OH)2 is made by heating strontium carbonate or strontium sulfide with steam at temperatures around 500 to 600°C: SrCO3 + H2O → Sr(OH)2 + CO2 SrS + 2H2O → Sr(OH)2 + H2S. - Flammability and Explosibility Not classified
- Purification Methods Crystallise the hydroxide from hot water (2.2mL/g) by cooling to 0o.
Strontium hydroxide Preparation Products And Raw materials
Raw materials
Preparation Products
Global(151)Suppliers
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12810
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:factory@coreychem.com
- Country:China
- ProdList:29808
- Advantage:58
-
Supplier:
SIMAGCHEM CORP
- Tel: +86-13806087780
- Email:sale@simagchem.com
- Country:China
- ProdList:17365
- Advantage:58
-
Supplier:
Hubei Ipure Biology Co., Ltd
- Tel: +8613367258412
- Email:ada@ipurechemical.com
- Country:China
- ProdList:10244
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
ANHUI WITOP BIOTECH CO., LTD
- Tel: +8615255079626
- Email:eric@witopchemical.com
- Country:China
- ProdList:23541
- Advantage:58
-
Supplier:
Baoji Guokang Healthchem co.,ltd
- Tel:+8615604608665<br/>15604608665
- Email:dominicguo@gk-bio.com
- Country:CHINA
- ProdList:9414
- Advantage:58
18480-07-4, Strontium hydroxide Related Search:
- CATALASE Strontium ranelate Strontium oxide Aluminum hydroxide Cobalt(II) hydroxide STRONTIUM Sodium hydroxide Calcium hydroxide Barium hydroxide Magnesium hydroxide Strontium carbonate Strontium sulfate Strontium fluoride Ammonium hydroxide Strontium chromate Strontium chloride STRONTIUM ACETATE Strontium chloride hexahydrate
- Strontium Salts
- Metal and Ceramic Science
- Materials Science
- Inorganic Bases
- Chemical Synthesis
- Synthetic Reagents
- Strontium Salts
- Salts
- Inorganic BasesMetal and Ceramic Science
- Chemical Synthesis
- Inorganics
- Synthetic Reagents
- 日用化工
- 无机碱
- 其他化工产品
- 其它原料及中间体
- 中间体产品
- 无机化工
- 氢氧化物
- 无机化学
- 化工中间体
- 有机中间体
- 无机盐
- Inorganic Bases
- Synthetic Reagents
- H2O2SrH18O10Sr
- H2OSr
- 氢氧化锶(无水)
- 氢氧化锶,95.00%
- 医药级无水氢氧化锶
- 氢氧化锶
- 18480-07-4
- StrontiuM hydroxide,95.00%
- Strontium hydroxide ISO 9001:2015 REACH
- StrontiuM hydroxide 94%
- Einecs 242-367-1
- Strontium dihydoxide
- Stronitium hydroxide
- STRONTIUM HYDROXIDE, 99.998%
- STRONTIUM HYDROXIDE
- STRONTIUM (II) HYDROXIDE
- strontiumhydroxide,anhydrous
- strontiumhydroxide(sr(oh)2)
- strontiumdihydroxide
- Strontium hydroxide (Sr(OH)2)
- Sr(OH)2