ChemicalBook > CAS DataBase List > Beraprost
Beraprost
Beraprost
- CAS No.88430-50-6
- Chemical Name:Beraprost
- CBNumber:CB8746083
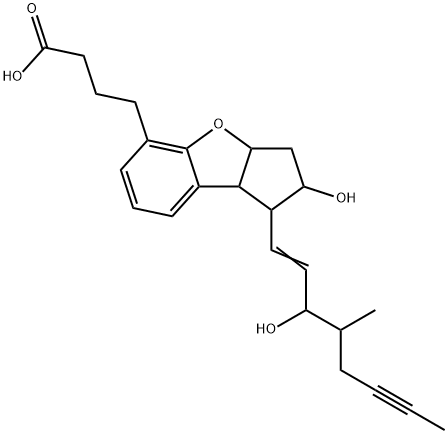
- Molecular Formula:C24H30O5
- Formula Weight:398.49
- MOL File:88430-50-6.mol
Beraprost Property
- Boiling point 572.1±50.0 °C(Predicted)
- Density 1.254±0.06 g/cm3(Predicted)
- pka 4.76±0.10(Predicted)
- FDA UNII 35E3NJJ4O6
- ATC code B01AC19
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
Beraprost Chemical Properties,Usage,Production
- Uses Platelet aggregation inhibitor; stable analog of Prostacyclin. Antithrombotic; vasodilator (peripheral).
- Mechanism of action Beraprost is a chemically stable, oral form of prostacyclin that is readily absorbed from GI tract. Like natural prostacyclin, beraprost dilates blood vessels, prevents platelet aggregation, and prevents proliferation of smooth muscle cells surrounding blood vessels. It may be an important treatment for early stage PVD and for early stage pulmonary hypertension. Intermittent oral doses of beraprost, however, do not seem to provide the consistent blood levels necessary to treat the advanced stages of pulmonary hypertension.
- Clinical Use Beraprost is an oral formulation of a prostacyclin analog for the treatment of early stage pulmonary hypertension as well as early stage PVD.
- Side effects Adverse effects include headache, flushing, jaw pain, and diarrhea.
- Enzyme inhibitor This orally active epoprostenol analogue (FW = 398.50 g/mol; CAS 88475- 69-8; Soluble to 25 mM in DMSO), also named TRA-418 and 2,3,3a,8b- tetrahydro-2-hydroxy-1- (3-hydroxy-4-methyl-1-octen-6-yn-1-yl) -1H- cyclopenta[b]benzofuran-5-butanoic acid, is a potent agonist for the Prostacyclin Receptor, a member of the G-protein coupled receptor family. Prostacyclin, the major product of cyclooxygenase in macrovascular endothelium, elicits a potent vasodilation and inhibition of platelet aggregation through binding to this receptor. Beraprost potently inhibits ADP-induced platelet aggregation (pIC50 = 8.26) and P-selectin expression in vitro (pIC50 = 8.56). It also increases vasodilation and reduces pulmonary hypertension in vivo. TRA-418 inhibited platelet GPIIb/IIIa activation as well as induction of P-selectin expression by adenosine 5'-diphosphate, Thrombin Receptor Agonist Peptide 1-6 (Ser-Phe-Leu-Leu-Arg-Asn-NH2), and U-46619 in the presence of epinephrine. TRA-418 also inhibits platelet aggregation induced by those platelet-stimulants in Ca2+-chelating anticoagulant, citrate and in nonchelating anticoagulant, d-phenylalanyl-l- prolyl-l-arginyl-chloromethyl ketone (PPACK). The TP-receptor antagonist SQ-29548 inhibited only U-46619+epinephrine-induced GPIIb/IIIa activation, P-selectin expression, and platelet aggregation. The IP-receptor agonist beraprost sodium inhibited platelet activation. Beraprost also inhibited platelet aggregation induced by platelet stimulants we tested in citrate and in PPACK. The GPIIb/IIIa inhibitor abciximab blocked GPIIb/IIIa activation and platelet aggregation. However, abciximab showed slight inhibitory effects on P-selectin expression. TRA-418 is more advantageous as an antiplatelet agent than TP-receptor antagonists or IP- receptor agonists separately used. TRA-418 showed a different inhibitory profile from abciximab in the effects on P-selectin expression.
Beraprost Preparation Products And Raw materials
Raw materials
Preparation Products
Global(47)Suppliers
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
Career Henan Chemica Co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:laboratory@coreychem.com
- Country:China
- ProdList:30231
- Advantage:58
-
Supplier:
AFINE CHEMICALS LIMITED
- Tel:+86-0571-85134551
- Email:sales@afinechem.com
- Country:China
- ProdList:15351
- Advantage:58
-
Supplier:
Hefei Hirisun Pharmatech Co., Ltd
- Tel: +8615056975894
- Email:shawn@hirisunpharm.com
- Country:CHINA
- ProdList:9911
- Advantage:58
-
Supplier:
Wuhan Golt Biotech Co., Ltd.
- Tel: +8615389281203
- Email:maria@goltbiotech.com
- Country:China
- ProdList:970
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418684<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:25356
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:
- Email:support@targetmol.com
- Country:United States
- ProdList:38630
- Advantage:58
-
Supplier:
Protheragen-ING
- Tel: +16313385890
- Email:info@protheragen-ing.com
- Country:United States
- ProdList:3868
- Advantage:58
-
Supplier:
Shaanxi Cuikang Pharmaceutical Technology Co., Ltd
- Tel: +8618791163155
- Email:18791163155@163.com
- Country:China
- ProdList:3558
- Advantage:58
-
Supplier:
XIAMEN AMITY INDUSTRY AND TRADE CO., LTD.
- Tel: +8618950047208
- Email:ellena@amitychem.com
- Country:China
- ProdList:43416
- Advantage:58
88430-50-6, BeraprostRelated Search:
- 前列腺素系列
- 药物
- 消化性溃疡治疗药
- 消化系统用药
- 化合物 T30426
- 贝拉普罗斯特
- 贝前列环素
- 2,3,3Α,8B-四氢-2-羟基-1-(3-羟基-4-甲基-1-辛烯-6-炔基)-1 H-环戊[B]苯并呋喃-5-丁酸
- 2,3,3A-8B-四氢-2-羟基-1-(3-羟基-4-甲基-1-辛烯-6-炔基)-1H-环戊并[B]苯并呋喃-5-丁酸
- 贝前列素
- 88430-50-6
- JWH-018 Impurity 5(N-Pentyl-5-carboxylic Acid)
- 1H-Cyclopenta[b]benzofuran-5-butanoic acid, 2,3,3a,8b-tetrahydro-2-hydroxy-1-(3-hydroxy-4-methyl-1-octen-6-yn-1-yl)-
- 4-[(1R,2R,3aS,8bS)-2-hydroxy-1-[(E,3S)-3-hydroxy-4-methyloct-1-en-6-ynyl]-2,3,3a,8b-tetrahydro-1H-cyclopenta[b]benzofuran-5-yl]butanoic acid
- Domer
- Befaprost
- 2,3,3α,8b-Tetrahydro-2-hydroxy-1-(3-hydroxy-4-methyl-1-octen-6-ynyl)-1H-cyclopenta[b]benzofuran-5-butanoic acid
- ML 1229
- MDL 201229
- 1H-Cyclopenta[b]benzofuran-5-butanoic acid, 2,3,3a,8b-tetrahydro-2-hydroxy-1-(3-hydroxy-4-methyl-1-octen-6-ynyl)-
- BERAPROST
- 2,3,3a,8b-Tetrahydro-2-hydroxy-1-(3-hydroxy-4-methyl-1-octen-6-ynyl)-1H-cyclopenta[b]benzofuran-5-butanoic acid
- Unii-35E3njj4o6
- Beraprostum [inn-latin]
- Beraprostum
- 88475-69-8 (Hydrochloride salt)
- 2-Hydroxy-1-(3-hydroxy-4-methyl-1-octen-6-ynyl)-2,3,3A,8B-tetrahydro-1H-cyclopenta(B)benzofuran-5-butanoic acid