ChemicalBook > CAS DataBase List > 1,1-Diethoxybutane
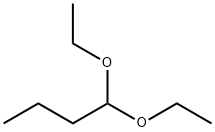
1,1-Diethoxybutane
1,1-Diethoxybutane
- CAS No.3658-95-5
- Chemical Name:1,1-Diethoxybutane
- CBNumber:CB8388533
- Molecular Formula:C8H18O2
- Formula Weight:146.23
- MOL File:3658-95-5.mol
1,1-Diethoxybutane Property
- Boiling point 143°C/760mmHg
- Density 0.829 g/mL at 20 °C
- refractive index n20/D1.396
- Flash point 30 °C
- storage temp. Flammables area
- form Liquid
- color Clear colorless
- BRN 1697910
- LogP 2.041 (est)
- NIST Chemistry Reference Butane, 1,1-diethoxy-(3658-95-5)
- EPA Substance Registry System Butane, 1,1-diethoxy- (3658-95-5)
Safety
- Hazard Codes :Xi
- Risk Statements :10-36/38
- Safety Statements :26-37/39-16
- RIDADR :UN 1993C 3 / PGIII
- WGK Germany :3
- HS Code :29329990
-
NFPA 704:
3 2 0
-
Symbol(GHS)


- Signal wordWarning
- Hazard statements H226-H315-H319
- Precautionary statements P305+P351+P338
1,1-Diethoxybutane Chemical Properties,Usage,Production
- Chemical Properties Clear colorless liquid
- Uses 1,1-Diethoxybutane-d10 is a deuterated labeled 1,1-Diethoxybutane[1].
-
Preparation
To a three-necked flask equipped with an air-tight mechanical stirrer, dropping funnel, reflux condenser, and drying tube, are added 3.0 gm (1.25 gm-atom) magnesium turnings, 50 ml of dry ether, and a small crystal of iodine. Then 5 gm of rt-butyl bromide is added dropwise until 171.0 gm (1.25 mole) has been added. The reaction takes about ½ - l h r if the reaction mixture is cooled. The solution is refluxed for ½ hr, cooled to 50°C, and then 148 gm (1.0 mole) of triethyl orthoformate is added dropwise over a ½-hr period. The reaction mixture is refluxed for 16 hr, crushed ice added to decompose the excess Grignard reagent, the ether separated and washed with water. The water layer is added to a separatory funnel containing 200 ml of ether, treated with acetic acid to pH 7.0, shaken, and the ether separated. The latter ether layer is washed with 10% aqueous sodium carbonate, water, and dried. The latter water layer is extracted twice again with ether (200 ml). The combined ether layers are dried over potassium carbonate and fractionated to afford 128 gm (80%), b.p. 143°-144°.
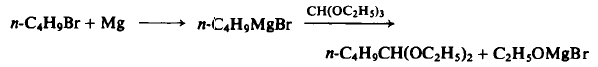
- References [1] Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019 Feb;53(2):211-216. DOI:10.1177/1060028018797110
1,1-Diethoxybutane Preparation Products And Raw materials
Raw materials
Preparation Products
Global(52)Suppliers
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-029-81138252<br/>+86-18789408387
- Email:1057@dideu.com
- Country:China
- ProdList:3922
- Advantage:58
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
Dayang Chem (Hangzhou) Co.,Ltd.
- Tel:+86-0571-88938639<br/>+8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:52849
- Advantage:58
-
Supplier:
GIHI CHEMICALS CO.,LIMITED
- Tel: +8618058761490
- Email:info@gihichemicals.com
- Country:China
- ProdList:49984
- Advantage:58
-
Supplier:
Aladdin Scientific
- Tel:
- Email:tp@aladdinsci.com
- Country:United States
- ProdList:52924
- Advantage:58
-
Supplier:
XIAMEN AMITY INDUSTRY AND TRADE CO., LTD.
- Tel: +8618950047208
- Email:ellena@amitychem.com
- Country:China
- ProdList:43416
- Advantage:58
-
Supplier:
Amadis Chemical Company Limited
- Tel:571-89925085
- Email:sales@amadischem.com
- Country:China
- ProdList:131957
- Advantage:58
-
Supplier:
Mainchem Co., Ltd.
- Tel:--
- Email:sarah@mainchem.com
- Country:China
- ProdList:6567
- Advantage:58
- Supplier: J & K SCIENTIFIC LTD.
- Tel: 18210857532
- Email:jkinfo@jkchemical.com
- Country:China
- ProdList:96815
- Advantage:76
3658-95-5, 1,1-DiethoxybutaneRelated Search:
- DIETHOXYMETHANE Diethyl carbonate Diethoxymethyl acetate 2-Ethoxyphenol Polyoxymethylene Butyraldehyde Bromoacetaldehyde diethyl acetal Mefenpyr-diethyl Diethyl malonate 2-Ethoxyethanol Butyl ethyl ether Diethyl phthalate POLY(VINYL BUTYRAL) Ethoxyquin Acetal Amygdalin Diethyl ether PHENYLETHYL ACETAL
- 高端化学
- 丁醛二乙缩醛
- 1,1-二乙氧基丁烷
- 3658-95-5
- Butane, 1,1-diethoxy-
- 1,1-DIETHOXYBUTANE
- Butanal diethyl acetal
- BUTYRALDEHYDE DIETHYLACETAL, 97+%
- butyraldehyde ethyl acetal
- BUTRALDEHYDE DIETHYL ACETAL
- BUTYRALDEHYDE DIETHYL ACETAL
- n-Butyraldehyde diethyl acetal
- Butylaldehyde diethyl acetal