ChemicalBook > CAS DataBase List > Metenolone
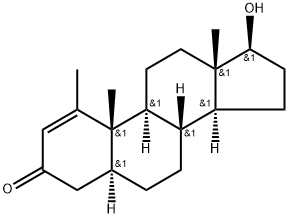
Metenolone
Metenolone
- CAS No.153-00-4
- Chemical Name:Metenolone
- CBNumber:CB8335436
- Molecular Formula:C20H30O2
- Formula Weight:302.46
- MOL File:153-00-4.mol
Metenolone Property
- Melting point 149.5-152°; mp 160-161° (Popper)
- alpha D +58.9°
- Boiling point 430.4±45.0 °C(Predicted)
- Density 1.084±0.06 g/cm3(Predicted)
- pka 15.08±0.70(Predicted)
- CAS DataBase Reference 153-00-4(CAS DataBase Reference)
- FDA UNII 9062ZT8Q5C
- ATC code A14AA04
Safety
- DEA Controlled Substances :CSCN: 4000
CSA SCH: Schedule III
NARC: No
-
Symbol(GHS)


- Signal wordDanger
- Hazard statements H351-H302-H362-H360
- Precautionary statements P201-P202-P281-P308+P313-P405-P501-P264-P270-P301+P312-P330-P501-P201-P260-P263-P264-P270-P308+P313
Metenolone Chemical Properties,Usage,Production
- Originator Primobolan ,Schering ,W. Germany,1961
- Uses Methenolone is an anabolic steroid. This is a controlled substance.
- Definition ChEBI: Methenolone is a 3-hydroxy steroid. It has a role as an androgen.
-
Manufacturing Process
8.42 ml of methyl iodide are slowly added dropwise at room temperature with
stirring in a nitrogen atmosphere to 3.067 g of magnesium turnings and 107
ml of absolute ether. After about 30 minutes, 185 ml of absolute
tetrahydrofuran are slowly introduced and then liquid is distilled off until a
boiling point of 62°C is reached. After cooling to room temperature, 613 mg
of cuprous chloride are added and then 10 g of ?1,4,6-androstatrien-17β-ol-3-
one-17-acetate in 110 ml of tetrahydrofuran slowly introduced. After 30
minutes reaction time, the whole is cooled to 0C, the excess of Grignard
reagent decomposed with saturated ammonium chloride solution, the product
diluted with ether and the aqueous phase separated. The ethereal phase is
washed consecutively with aqueous sodium thiosulfate solution, saturated
ammonium chloride solution and water. It is dried over sodium sulfate and
evaporated to dryness under vacuum. The residue is dissolved in 40 ml of
pyridine and 20 ml of acetic anhydride and the solution kept for 16 hours at
room temperature. It is then stirred into ice water and the precipitate filtered
with suction, dried and recrystallized from isopropyl ether. 1α-Methyl-?4,6-
androstadien-17β-ol-3-one-17-acetate is obtained. MP 156°C to 157°C; [α]D25
= -33.8° (in CHCl3; c = 0.9). Yield 65-70% of the theoretical.
4.67 g of 1α-methyl-?4,6-androstadien-17β-ol-3-one-17-acetate are dissolved in 273 ml of methanol and, after the addition of 350 mg of 10% palladium on calcium carbonate catalyst, hydrogenated until 1 mol equivalent of hydrogen has been taken up. After filtering off the catalyst, the solution is treated with 150 ml of 2N-hydrochloric acid and evaporated under vacuum to about 1/3 of the volume. The whole is then diluted with water and extracted with ether. The ethereal solution is washed with water until neutral, dried over sodium sulfate and evaporated. The crude product is heated on a steam bath for 90 minutes in 10 ml of pyridine and 10 ml of acetic anhydride. Extraction with ether is then carried out and the ethereal phase washed until neutral with water. The crude crystalline 1α-methyl-?4-androsten-17β-ol-3-one-17- acetateobtained after drying and evaporation of the solution, melts at 122°C to 129°C. Yield 98% of the theoretical.
1α-Methyl-?4-androsten-17β-ol-3-one-17-acetate when purified by recrystallization from isopropyl ether melts at 138°C to 139°C. - Therapeutic Function 17β-Hydroxy-1β-methyl-5α-androst-l-ene-3-one acetate
Metenolone Preparation Products And Raw materials
Raw materials
Preparation Products
Global(75)Suppliers
-
Supplier:
Hebei Yanxi Chemical Co., Ltd.
- Tel: +8617531153977
- Email:allison@yan-xi.com
- Country:China
- ProdList:5854
- Advantage:58
-
Supplier:
Nantong Guangyuan Chemicl Co,Ltd
- Tel: +undefined17712220823
- Email:admin@guyunchem.com
- Country:China
- ProdList:615
- Advantage:58
-
Supplier:
hebei hongtan Biotechnology Co., Ltd
- Tel:+852-6619 3215<br/>+86-17731935328
- Email:sales03@chemcn.cn
- Country:China
- ProdList:970
- Advantage:58
-
Supplier:
Hubei XinRunde Chemical Co., Ltd.
- Tel:+8615102730682
- Email:bruce@xrdchem.cn
- Country:CHINA
- ProdList:566
- Advantage:55
-
Supplier:
SHANDONG ZHI SHANG CHEMICAL CO.LTD
- Tel: +86 18953170293
- Email:sales@sdzschem.com
- Country:China
- ProdList:2930
- Advantage:58
-
Supplier:
Hebei Miaobian Biotechnology Co., Ltd
- Tel: +8617733850068
- Email:
- Country:CHINA
- ProdList:967
- Advantage:58
-
Supplier:
Qiuxian Baitai New Material Co., LTD
- Tel: +8618330912755
- Email:sale2@hbyalin.com
- Country:China
- ProdList:1658
- Advantage:58
-
Supplier:
Zhejiang J&C Biological Technology Co.,Limited
- Tel:+1-2135480471<br/>+1-2135480471
- Email:sales@sarms4muscle.com
- Country:China
- ProdList:10473
- Advantage:58
-
Supplier:
GIHI CHEMICALS CO.,LIMITED
- Tel: +8618058761490
- Email:info@gihichemicals.com
- Country:China
- ProdList:49984
- Advantage:58
-
Supplier:
Hebei Miaoyin Technology Co.,Ltd
- Tel:+86-17367732028<br/>+86-17367732028
- Email:kathy@hbyinsheng.com
- Country:China
- ProdList:3512
- Advantage:58
153-00-4, MetenoloneRelated Search:
- Methyl acetate Mestanolone Atipamezole Tribenuron methyl METSULFURON METHYL N,N-Dimethylallylamine METYRAPONE Methyl cyclopentenolone 6-Methyl-5-hepten-2-one METHYLCYCLOPENTADIENE DIMER Dehydronandrolone Acetate Nandrolone Decanoate Kresoxim-methyl Nandrolone CHLOROPHOSPHONAZO III ALLYL METHYL ETHER Methyl salicylate Thiophanate-methyl
- Steroid and Hormone
- Steroids
- Finished Steroid and Hormone
- Pharmaceuticals
- Intermediates & Fine Chemicals
- 激素类
- 医药原料
- 原料
- 医药中间体
- 乙腈中美替诺龙
- 美替诺龙
- 美替诺龙,1.0毫克/毫升
- 甲基异睾酮
- 17beta-羟基-1-甲基-5alpha-雄甾-1-烯-3-酮
- 153-00-4
- Methenolone Acetate, Primobolan 50mg Tablets
- Methenolone Acetate, Primobolan 25mg Tablets
- Metenolone base
- 1(5α)-ANDROSTEN-1β-METHYL-17β-OL-3-ONE
- (5S,8R,9S,10S,13S,14S,17S)-17-hydroxy-1,10,13-trimethyl-4,5,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one
- Metenolone Acetat
- Metenolone USP/EP/BP
- Androst-1-en-3-one, 17-hydroxy-1-methyl-, (5α,17β)-
- Methenolon
- 1-Methyl-Δ1-androsten- 17β-ol-3-one
- 1-Methyl-5α-androst-1-en-17β-ol-3-one
- (5α,17β)-17-Hydroxy-1-Methylandrost-1-en-3-one
- Methenolone Tablet
- Methenolone Base
- Methenolone, 1.0 mg/mL
- (5S)-17-hydroxy-1,10,13-trimethyl-4,5,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one
- Metenolone Chemical
- Androst-1-en-3-one, 17-hydroxy-1-methyl-, (5a,17b)-
- 5a-Androst-1-en-3-one, 17b-hydroxy-1-methyl-
- 17-Hydroxy-1,10,13-trimethyl-4,5,6,7,8,9,10,11,12,13, 14,15,16,17-tetradecahydro-cyclopenta[a]phenanthren-3-one
- Androst-1-en-3-one, 17-hydroxy-1-methyl-, (5a,17b)- (9CI)
- 5a-Androst-1-en-3-one, 17b-hydroxy-1-methyl- (6CI, 7CI, 8CI)
- 1-Methyl-D1-androsten-17b-ol-3-one
- 17b-Hydroxy-1-methyl-5a-androst-1-en-3-one
- MethenoloneAcetate434-05-9/Base
- 17beta-hydroxy-1-methyl-5alpha-androst-1-en-3-one
- 1,5ALPHA-ANDROSTEN-1-METHYL-17BETA-OL-3-ONE
- 1(5-ALPHA)-ANDROSTEN-1-BETA-METHYL-17-BETA-OL-3-ONE
- metenolone
- METHENOLONE
- (5S,8R,9S,10S,13S,14S,17S)-17-hydroxy-1,10,13-triMethyl-4,5,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one