ChemicalBook > CAS DataBase List > Glimepiride
Glimepiride
Glimepiride
- CAS No.93479-97-1
- Chemical Name:Glimepiride
- CBNumber:CB8318552
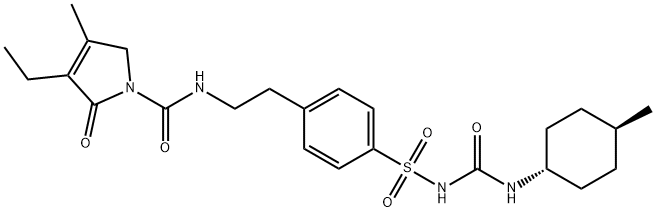
- Molecular Formula:C24H34N4O5S
- Formula Weight:490.62
- MOL File:93479-97-1.mol
Glimepiride Property
- Melting point 212.2-214.5 °C
- Density 1.29±0.1 g/cm3(Predicted)
- storage temp. room temp
- solubility DMSO: >10 mg/mL
- pka 5.10±0.10(Predicted)
- form solid
- color white
- Merck 14,4440
- BCS Class 2
- CAS DataBase Reference 93479-97-1(CAS DataBase Reference)
- FDA UNII 6KY687524K
- NCI Drug Dictionary Amaryl
- ATC code A10BB12
- EPA Substance Registry System 1H-Pyrrole-1-carboxamide, 3-ethyl-2,5-dihydro-4-methyl-N-[2-[4-[[[[(trans- 4-methylcyclohexyl)amino]carbonyl]amino]sulfonyl]phenyl]ethyl]-2-oxo- (93479-97-1)
- UNSPSC Code 41116107
- NACRES NA.77
Safety
- Hazard Codes :Xn,Xi
- Risk Statements :21-36/38-46-62-63
- Safety Statements :25-26-36/37-53
- WGK Germany :3
- RTECS :UX9363950
- HS Code :2935904000
-
NFPA 704:
0 4 0
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H361
- Precautionary statements P201-P202-P280-P308+P313-P405-P501
Glimepiride Price
More Price(5)
- Brand: Sigma-Aldrich(India)
- Product number: PHR1617
- Product name : Glimepiride
- Purity: Pharmaceutical Secondary Standard; Certified Reference Material
- Packaging: 1G
- Price: ₹15966.88
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: G2295
- Product name : Glimepiride
- Purity: ≥98% (HPLC), solid
- Packaging: 50MG
- Price: ₹17060.2
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: G2295
- Product name : Glimepiride
- Purity: ≥98% (HPLC), solid
- Packaging: 250MG
- Price: ₹76283.78
- Updated: 2022/06/14
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: G0395
- Product name : Glimepiride
- Purity:
- Packaging: 1G
- Price: ₹8400
- Updated: 2022/05/26
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: G0395
- Product name : Glimepiride
- Purity:
- Packaging: 5G
- Price: ₹11800
- Updated: 2022/05/26
- Buy: Buy
Glimepiride Chemical Properties,Usage,Production
- Description Glimepiride (original trade name Amaryl) is an orally available medium-to-long-acting sulfonylurea antidiabetic drug. It is sometimes classified as either the first third-generation sulfonylurea, or as second-generation. Like all sulfonylureas, glimepiride acts as an insulin secretagogue. It lowers blood sugar by stimulating the release of insulin from functioning pancreatic beta cells and by increasing sensitivity of peripheral tissues to insulin. Glimepiride likely binds to ATP-sensitive potassium channel receptors on the pancreatic cell surface, reducing potassium conductance and causing depolarization of the membrane. Membrane depolarization stimulates calcium ion influx through voltage-sensitive calcium channels. This increase in intracellular calcium ion concentration induces the secretion of insulin. Glimepiride is mainly used to treat patients with type 2 diabetes and can also decrease the chances that someone will develop complications of type 2 diabetes, such as kidney damage, blindness, nerve problems, loss of limbs, sexual function problems and heart attack or stroke. The drug was approved by the FDA in 1995 and is manufactured by Sanofi-Aventis. It can be used along with proper diet and exercise program and may also be used alone or with other antidiabetic medicines if need. The drug is available only with your doctor's prescription.
-
References
1. https://en.wikipedia.org/wiki/Glimepiride
2. http://www.webmd.com/drugs/2/drug-12271/glimepiride-oral/details
3. https://www.drugs.com/cdi/glimepiride.html
4. http://www.medicinenet.com/glimepiride/article.htm
5. http://www.everydayhealth.com/drugs/glimepiride
6. http://www.emedicinehealth.com/drug-glimepiride/article_em.htm
7. https://www.ghc.org/kbase/topic.jhtml?docId=d03864a1
8. http://www.emedicinehealth.com/drug-glimepiride/article_em.htm
9. http://drugs.healthgrove.com/l/3454/Glimepiride - Description Glimepiride, the first of a new generation of sulfonylurea drugs, was introduced in Sweden in 1995 as a first-line therapy to lower blood glucose in patients with type II diabetes. Sulfonylureas exert their hypoglycemic function primarily by direct stimulation of insulin secretion in glucose-insensitive pancreatic β-cells and GLUT translocation in insulin-resistant fat and muscle cells. Once-daily, orally administered glimepiride in diabetes patients showed a more rapid and longer lasting glucose-lowering effect than the commonly used agent glibenclamide. Glimepiride can be used either as a monotherapy or in combination with insulin.
- Chemical Properties White Cyrstalline Solid
- Originator Hoechst Marion Roussel (Germany)
- Uses A sulfonylurea hypoglycemic agent. Used as an antidiabetic
- Uses Glimepiride induces the PI3 kinase (PI3K) and Akt pathway, along with insulin receptor substrate-1/2 and endothelial nitric oxide synthase. Glimepiride also increases osteoblast proliferation and differentiation, which is thought to be related to its ability to activate the PI3K and Akt pathway. Furthermore, Glimepiride enhances intrinsic peroxisome proliferator-activated receptor γ activity. Glimepiride also increases protein expression of glucose transports 1 and 4, and is a potent KIR channel blocker. Potent Kir6 (KATP) channel blocker and anti-diabetic agent. Inhibits pinacidil-activated cardiac Kir6 channels with an IC50 of 6.8 nM.
- Uses For concomitant use with insulin for the treatment of noninsulin-dependent (type 2) diabetes mellitus.
- Uses anticonvulsant
- Definition ChEBI: Glimepiride is a sulfonamide, a N-acylurea and a N-sulfonylurea. It has a role as a hypoglycemic agent and an insulin secretagogue.
- Manufacturing Process By heating of a mixture of 3-ethyl-4-methyl-2-pyrrolone and 2- phenylethylisocyanate at 150°C is obtained 3-ethyl-4-methyl-2-oxo-3- pyrroline-1-(N-2-phenylethyl)-carboxamide, melting point 106°-108°C. Then the carboxamide are introduced in portions at 30°C into chlorosulfonic acid, and agitated for 1 hour at 40°C. The sulfochloride (melting point 172-175°C), introduced into concentrated ammonia, and heated for 30 min on a steam bath. The mixture of sulfonamide obtained (melting point 180°-182°C), of acetone and K2CO3 are refluxed with agitation for 6 hours. Subsequently the cyclohexyl isocyanate are added dropwise, and agitation is continued for 6 hours at boiling temperature. After standing overnight, the product is filtered, the crystals obtained are treated with dilute hydrochloric acid, and again filtered. It is prepared N-(4-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1- carboxamido)ethyl]benzenesulfonyl)-N'-cyclohexyl urea; melting point 185°- 187°C (from acetone) (Glimepiride).
- brand name Amaryl (Sanofi Aventis).
- Therapeutic Function Oral hypoglycemic
- General Description Glimepiride, 1-[[p-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido)ethyl]phenyl]sulfonyl]-3-(trans-4-methylcyclohexyl)urea (Amaryl), is very similarto glipizide with the exception of their heterocyclic rings.Instead of the pyrazine ring found in glipizide, glimepiridecontains a pyrrolidine system. It is metabolized primarilythrough oxidation of the alkyl side chain of the pyrrolidine,with a minor metabolic route involving acetylation of theamine.
- General Description Glimepiride is 3-ethyl-2,5-dihydro-4-methyl-N-[2-[4-[[[[(trans-4-methylcyclohexyl)amino]-carbonyl]amino]sulfonyl]phenyl]ethyl]-2-oxo-1H-pyrrole-1-carboxamide; thiscompound can also be named as the urea—see precedingdiscussion (Amaryl, generic). Combinations are availablewith rosiglitazone in the United States (Avandaryl tablets;mg glimepiride/mg rosiglitazone as maleate salt: 1/4,2/4, 4/4, 2/8, 4/8); and with pioglitazone (Duetact tablets;mg glimepiride/ mg pioglitazone as hydrochloride salt:2/30, 4/30).
- Biological Activity Potent K ATP channel blocker and anti-diabetic agent. Inhibits pinacidil-activated cardiac K ATP channels with an IC 50 of 6.8 nM.
- Biochem/physiol Actions Glimepiride is a potent blocker of cardiac KATP channels activated by pinacidil with an IC50 of 6.8 nM.
- Clinical Use Non-insulin dependent diabetes mellitus
- Veterinary Drugs and Treatments Glimepiride may potentially be a useful adjunct in the treatment of non-insulin dependent diabetes mellitus (NIDDM) in cats. Its duration of action in humans allows it to be dosed once daily, which could be of benefit in cats. It may also have fewer side effects than glipizide in cats.
-
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: effects enhanced by NSAIDs.
Antibacterials: effects enhanced by chloramphenicol, sulphonamides, tetracyclines and trimethoprim; effect reduced by rifamycins.
Anticoagulants: effect possibly enhanced by coumarins; also possibly changes to INR.
Antifungals: concentration increased by fluconazole and miconazole and possibly voriconazole.
Lipid-regulating drugs: possibly additive hypoglycaemic effect with fibrates.
Sulfinpyrazone: enhanced effect of sulphonylureas. -
Metabolism
The drug is extensively metabolised in the liver to two
main metabolites. The cytochrome P450 isoenzyme
CYP2C9 is involved in the formation of a hydroxy
derivative, which is further metabolised to a carboxy
derivative by cytosolic enzymes.
About 60% of a dose is eliminated in the urine and 40% in the faeces.
Glimepiride Preparation Products And Raw materials
Raw materials
Preparation Products
Global(670)Suppliers
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12809
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5868
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531151365
- Email:mina@chuanghaibio.com
- Country:China
- ProdList:18137
- Advantage:58
-
Supplier:
Hebei Kingfiner Technology Development Co.Ltd
- Tel:+86-15532196582<br/>+86-15373005021
- Email:lisa@kingfinertech.com
- Country:China
- ProdList:3005
- Advantage:58
-
Supplier:
Shaanxi TNJONE Pharmaceutical Co., Ltd
- Tel:+86-17396673057
- Email:linda@tnjone.com
- Country:China
- ProdList:1143
- Advantage:58
-
Supplier:
Capot Chemical Co.,Ltd.
- Tel:+86-(0)57185586718<br/>+86-13336195806
- Email:sales@capot.com
- Country:China
- ProdList:29730
- Advantage:60
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Hubei XinRunde Chemical Co., Ltd.
- Tel:+8615102730682
- Email:bruce@xrdchem.cn
- Country:CHINA
- ProdList:566
- Advantage:55
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418679<br/>+8618949832763
- Email:info@tnjchem.com
- Country:China
- ProdList:2986
- Advantage:55
93479-97-1, GlimepirideRelated Search:
- Methyl acrylate Methanol Basic Violet 1 N,N-Dimethylformamide Acetonitrile Methylparaben Paraquat dichloride Formamide Pyrrole Foramsulfuron Kresoxim-methyl PHENYL VALERATE Chlorantraniliprole Repaglinide Nateglinide Methyl rac trans-Hydroxy Glimepiride-D5,trans-Hydroxy Glimepiride Glimepiride sulfonamide,Des[(trans-4-methylcyclohexyl)amino]carbonyl Glimepiride
- 93479-97-1
- API's
- Voltage-gated Ion Channels
- Potassium Channel Modulators
- Monovalent Ion Channels
- Pharmaceuticals
- Intermediates & Fine Chemicals
- APIs
- Active Pharmaceutical Ingredients
- Sulfur & Selenium Compounds
- Heterocycles
- Chiral Reagents
- NEURONTIN
- API
- Diabetes Research
- 高纯化学试剂
- 药靶配体
- 合成有机化合物配体
- 添加剂
- 试剂
- 对照品
- 精细化工原料
- 抑制剂
- 医药体
- 化学试剂
- 化学原料
- 降糖原料
- 医药、农药及染料中间体
- 医药原料药 科研原料
- 药物杂质及中间体
- 医药原料药
- 医用原料
- 对照品-杂质对照品
- 医药原料药-科研原料
- 原料中间体-原料药
- 医用原料
- 对照品
- 生化试剂
- 医药 降糖药
- 化合物:原料药
- 原料
- 系列1
- 有机中间体
- 化工原料
- 小分子抑制剂
- 降血糖类
- 抗生素类
- 胰腺激素及其它调节血糖药
- 原料药
- 其他兽药标准品
- 抗生素
- 医药原料
- 药物
- 糖尿病治疗药
- 激素及其有关药物
- C24H34N4O5S
- C24H30D4N4O5S
- 93479-91-1