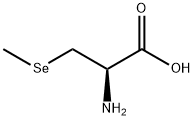
ChemicalBook > CAS DataBase List > Se-Methylselenocysteine
Se-Methylselenocysteine
Se-Methylselenocysteine
- CAS No.26046-90-2
- Chemical Name:Se-Methylselenocysteine
- CBNumber:CB8265719
- Molecular Formula:C4H9NO2Se
- Formula Weight:182.08
- MOL File:26046-90-2.mol
Se-Methylselenocysteine Property
- Melting point 177 °C(dec.)
- Boiling point 314.1±37.0 °C(Predicted)
- storage temp. Keep in dark place,Inert atmosphere,2-8°C
- solubility DMSO (Slightly), Water (Slightly)
- pka 2.17±0.10(Predicted)
- form Powder
- color White to slightly yellow
- InChI InChI=1S/C4H9NO2Se/c1-8-2-3(5)4(6)7/h3H,2,5H2,1H3,(H,6,7)/t3-/m0/s1
- InChIKey XDSSPSLGNGIIHP-VKHMYHEASA-N
- SMILES C(O)(=O)[C@H](C[Se]C)N
- LogP -0.043 (est)
- NCI Dictionary of Cancer Terms Se-methyl-seleno-L-cysteine
- FDA UNII TWK220499Z
Safety
- Hazard Codes :T,N
- Risk Statements :23/25-33-50/53
- Safety Statements :20/21-28-45-60-61-28A
- RIDADR :UN 3283 6.1/PG 2
- WGK Germany :3
- F :10-23
- HS Code :29319090
-
Symbol(GHS)



- Signal wordDanger
- Hazard statements H301+H331-H373-H410
- Precautionary statements P501-P273-P260-P270-P271-P264-P391-P314-P301+P310+P330-P304+P340+P311-P403+P233-P405
Se-Methylselenocysteine Price
More Price(1)
- Brand: TCI Chemicals (India)
- Product number: S0901
- Product name : Se-(Methyl)seleno-L-cysteine
- Purity:
- Packaging: 100MG
- Price: ₹8900
- Updated: 2022/05/26
- Buy: Buy
Se-Methylselenocysteine Chemical Properties,Usage,Production
- Description Se-methyl selenocysteine (MSC) is a naturally occurring selenium compoundwith favourable pharmacokinetic properties andth high peroral bioavailability in humans. MSC is synthesized by plants such as garlic, astragalus, onions and broccoli. Se-methylselenocysteine has been proven to have a chemo-preventive property, which is shown by several authors via various cell culture models and animal models. It has also been shown to have anti-carcinogenic properties by inducing cell cycle arrest and apoptotic cell death. It is considered a pro-drug because the compound per se is not toxic unless it is metabolized by the enzymes Kynurenine aminotransferase 1 (KYAT1), Kynurenine aminotransferase 3 (KYAT3) and cystathionine γ-lyase (CTH). Of these, KYAT1 plays a vital role in MSC metabolism.
- Chemical Properties white to slightly yellow powder
- Uses 2-Amino-3-methylselenyl propionic acid is an inhibitor of DMBA-induced mammary tumors.
- Definition ChEBI: Se-methyl-L-selenocysteine is an L-alpha-amino acid compound having methylselanylmethyl as the side-chain. It has a role as an antineoplastic agent. It is a Se-methylselenocysteine, a non-proteinogenic L-alpha-amino acid and a L-selenocysteine derivative. It is a conjugate base of a Se-methyl-L-selenocysteinium. It is a conjugate acid of a Se-methyl-L-selenocysteinate. It is an enantiomer of a Se-methyl-D-selenocysteine. It is a tautomer of a Se-methyl-L-selenocysteine zwitterion.
- benefits Se-methylselenocysteine (MSC) is a naturally occurring selenium compound with favorable pharmacokinetic properties and high peroral bioavailability in humans. MSC has been proven to have a chemo-preventive property, which several authors show via various cell culture models and animal models. It has also been shown to have anti-carcinogenic properties by inducing cell cycle arrest and apoptotic cell death. It is considered a pro-drug because the compound per se is not toxic unless it is metabolized by the enzymes Kynurenine aminotransferase 1 (KYAT1), Kynurenine aminotransferase 3 (KYAT3) and cystathionine γ-lyase (CTH). Of these, KYAT1 plays a vital role in MSC metabolism. KYAT1 is a PLP dependent enzyme whose main function is to cleave carbon-sulfur bonds. KYAT is a bi-functional enzyme that catalyzes transamination and β-elimination reactions with single substrates. KYAT1 metabolizes MSC into β-methylselenopyruvate (MSP) and methylselenol via transamination and β-elimination catalysis.
- Side effects Se-Methylselenocysteine (MSC) is a selenium (Se) supplement with antioxidant and anticancer effects, and its efficacy and safety are superior to selenomethionine supplements. However, Se may cause toxic reactions. Long-term dietary intake of Se compounds (500 μg Se per day) may cause adverse reactions such as endocrine disruption, immunity modulation, hepatotoxicity, and amyotrophic lateral sclerosis in humans. The acceptable daily intake (ADI) of MSC in human diet is estimated to be 3.4 μg/kg BW/day. MSC is reported to be less toxic and more bioactive than inorganic or other organic Se compounds. The reason may be that selenomethionine is nonspecifically incorporated into proteins as the amino acid methionine and further provides reversible Se storage in organs and tissues, while MSC is not easily incorporated into proteins and accumulates in the free pool after ingestion[1].
- References [1] HUI YANG Xudong J. Safety evaluation of Se-methylselenocysteine as nutritional selenium supplement: Acute toxicity, genotoxicity and subchronic toxicity[J]. Regulatory Toxicology and Pharmacology, 2014, 70 3: Pages 720-727. DOI:10.1016/j.yrtph.2014.10.014.
Se-Methylselenocysteine Preparation Products And Raw materials
Raw materials
Preparation Products
Global(223)Suppliers
-
Supplier:
Shangdong Yuanlitai Nutratech Co.,ltd
- Tel: +8618782242822
- Email:tatiana@yltnutra.com
- Country:China
- ProdList:8
- Advantage:58
-
Supplier:
Hebei Yanxi Chemical Co., Ltd.
- Tel: +8617531153977
- Email:allison@yan-xi.com
- Country:China
- ProdList:5854
- Advantage:58
-
Supplier:
BINBO BIOLOGICAL CO.,LTD
- Tel: +8618629063126
- Email:info@binbobiological.com
- Country:China
- ProdList:452
- Advantage:58
-
Supplier:
Chembon Pharmaceutical Co., Ltd.
- Tel:+86-28-8425-2981
- Email:
- Country:CHINA
- ProdList:722
- Advantage:55
-
Supplier:
Hubei XinRunde Chemical Co., Ltd.
- Tel:+8615102730682
- Email:bruce@xrdchem.cn
- Country:CHINA
- ProdList:566
- Advantage:55
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29858
- Advantage:58
-
Supplier:
Hubei xin bonus chemical co. LTD
- Tel:86-13657291602
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:22963
- Advantage:58
-
Supplier:
BOC Sciences
- Tel:+1-631-485-4226
- Email:inquiry@bocsci.com
- Country:United States
- ProdList:19552
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
26046-90-2, Se-MethylselenocysteineRelated Search:
- 26046-90-2
- food additives
- Cell Cycle Regulators
- Cell Cycle
- Antioxidant and Free Radical ScavengersApoptosis and Cell Cycle
- Nutrition Research
- Chemopreventive Agents
- Biochemicals Found in Plants
- AntioxidantCancer Research
- 大化工产品
- 营养强化补充剂/原料
- 食品营养强化剂
- 抑制剂
- 化学品定制
- 其它原料及中间体
- 生化试剂
- 化学和生化试剂-生化试剂
- 杂质对照品
- 营养剂
- 硒原料
- 食品原料
- 精细化工原料
- 氨基酸衍生物
- 膳食营养添加剂
- 对照品
- 化学试剂
- 医用原料
- 日用化学品
- 医药化工类
- 功能性物质-硒代蛋氨酸
- 食品添加剂
- 有机化工原料
- 其他化工产品
- 营养强化剂
- 原料药
- 氨基酸
- 医药原料药
- 胺
- 有机原料
- Specialty Synthesis
- Unnatural Amino Acid Derivatives
- Cysteine Derivatives
- Peptide Synthesis
- C4H9NO2SSe
- C4H9NO2SeHCl
- C4H9NO2Se
- L-硒甲基硒代半胱氮酸
- 水中硒代半胱氨酸溶液标准物质(NY/T 4353-2023)
- 水中甲基硒代半胱氨酸溶液标准物质
- 甲基硒代半胱氨酸检测标准品
- 甲基硒-L-半胱氨酸
- L-硒甲基硒代半胱氨酸(L-SEMC)
- SE-(甲基)硒基-<SMALL>L</SMALL>-半胱氨酸
- L-硒甲基硒代半胱氨酸
- SE-(甲基)硒基-L-半胱氨酸
- SE-甲基硒代-L-半胱氨酸
- 甲基硒代半胱胺酸
- 甲基硒代半胱氨酸