ChemicalBook > CAS DataBase List > Atorvastatin
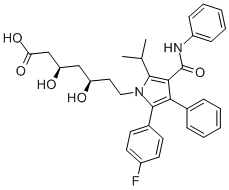
Atorvastatin
Atorvastatin
- CAS No.110862-48-1
- Chemical Name:Atorvastatin
- CBNumber:CB81269853
- Molecular Formula:C33H35FN2O5
- Formula Weight:558.64
- MOL File:110862-48-1.mol
Atorvastatin Property
- Boiling point 722.2±60.0 °C(Predicted)
- Density 1.23±0.1 g/cm3(Predicted)
- pka 4.29±0.10(Predicted)
- FDA UNII 36TN91XZ0V
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
Atorvastatin Chemical Properties,Usage,Production
- Definition ChEBI: Atorvastatin is a dihydroxy monocarboxylic acid that is a member of the drug class known as statins, used primarily for lowering blood cholesterol and for preventing cardiovascular diseases. It has a role as an environmental contaminant and a xenobiotic. It is an aromatic amide, a member of monofluorobenzenes, a statin (synthetic), a dihydroxy monocarboxylic acid and a member of pyrroles. It is functionally related to a heptanoic acid. It is a conjugate acid of an atorvastatin(1-).
- Clinical Use Hyperlipidaemia and hypercholesterolaemia
-
Drug interactions
Potentially hazardous interactions with other drugs
Anti-arrhythmics: concentration possibly increased
by dronedarone.
Antibacterials: azithromycin, erythromycin,
clarithromycin or fusidic acid possibly increased
risk of myopathy - avoid atorvastatin for at least
7 days after fusidic acid stopped; concentration
increased by clarithromycin - do not exceed 20 mg
of atorvastatin1
; avoid with telithromycin; increased
risk of myopathy with daptomycin; concentration
possibly reduced by rifampicin.
Anticoagulants: may transiently reduce anticoagulant effect of warfarin.
Antifungals: increased risk of myopathy with itraconazole - do not exceed 40 mg of atorvastatin1 ;
increased risk of myopathy with fluconazole, ketoconazole, posaconazole, voriconazole and possibly other imidazoles and triazoles - avoid.
Antivirals: increased risk of myopathy with atazanavir, boceprevir (reduce atorvastatin dose), and possibly darunavir, fosamprenavir, indinavir, lopinavir, ritonavir, saquinavir or tipranavir (max dose of atorvastatin 10 mg); concentration reduced by efavirenz and possibly etravirine; avoid with dasabuvir, ombitasvir, paritaprevir and telaprevir; possible increased risk of myopathy with ledipasvir - reduce atorvastatin dose; concentration increased by simeprevir - consider reducing atorvastatin dose.
Calcium channel blockers: concentration increased by diltiazem - increased risk of myopathy; concentration of verapamil increased also possible increased risk of myopathy - consider reducing atorvastatin dose.
Ciclosporin: increased risk of myopathy - do not exceed 10 mg of atorvastatin.1 Cobicistat: reduce atorvastatin dose.
Colchicine: possible increased risk of myopathy.
Grapefruit juice: concentration possibly increased.
Lipid lowering agents: increased risk of myopathy
with fibrates, gemfibrozil (avoid) and nicotinic acid. - Metabolism Atorvastatin undergoes extensive presystemic clearance in gastrointestinal mucosa and/or hepatic first-pass metabolism. Atorvastatin is metabolised by cytochrome P450 3A4 to ortho- and parahydroxylated derivatives and various beta-oxidation products. These products are further metabolised via glucuronidation. Approximately 70% of circulating inhibitory activity for HMG-CoA reductase is attributed to active metabolites. Atorvastatin is eliminated primarily in bile as active metabolites following hepatic and/or extrahepatic metabolism, but does not appear to undergo significant enterohepatic recirculation.
Atorvastatin Preparation Products And Raw materials
Raw materials
Preparation Products
Global(93)Suppliers
-
Supplier:
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
- Tel:+86-18600796368<br/>+86-18600796368
- Email:sales@sjar-tech.com
- Country:China
- ProdList:485
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Shanghai Zheyan Biotech Co., Ltd.
- Tel:18017610038
- Email:zheyansh@163.com
- Country:CHINA
- ProdList:3619
- Advantage:58
-
Supplier:
Biochempartner
- Tel:0086-13720134139
- Email:candy@biochempartner.com
- Country:CHINA
- ProdList:965
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:factory@coreychem.com
- Country:China
- ProdList:29808
- Advantage:58
-
Supplier:
HANGZHOU CLAP TECHNOLOGY CO.,LTD
- Tel:86-571-88216897,88216896<br/>13588875226
- Email:sales@hzclap.com
- Country:CHINA
- ProdList:6312
- Advantage:58
-
Supplier:
Xi'an MC Biotech, Co., Ltd.
- Tel:029-89275612<br/>+8618991951683
- Email:mcbio_sales@163.com
- Country:China
- ProdList:2251
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-029-89586680<br/>+86-18192503167
- Email:1026@dideu.com
- Country:China
- ProdList:7859
- Advantage:58
-
Supplier:
sgtlifesciences pvt ltd
- Tel: +8617013299288
- Email:dj@sgtlifesciences.com
- Country:China
- ProdList:12373
- Advantage:58
110862-48-1, AtorvastatinRelated Search:
- Atorvastatin ATORVASTATIN SODIUM Diphenyl ether Polyvinylpyrrolidone Simvastatin N-Methyl-2-pyrrolidone PHENYL VALERATE Povidone iodine Phenyl salicylate N-Vinyl-2-pyrrolidone Propane PHENYL RESIN Phenylacetic acid Atorvastatin calcium METHYL-2-PYRROLIDONE tert-Butyl (4R,6R)-2-[[[6-(2-4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxan-4-yl]acetate P-HYDROXY ATORVASTATIN, DISODIUM SALT Heptanoic acid
- Cardiovascular Agents
- 医药、农药及染料中间体
- 医药原料药
- 科研原料
- 化合物 T22248
- 阿托伐他汀杂质153
- (REL)-阿托伐他汀
- 阿伐他汀/阿托伐他汀
- 阿伐他汀, ?>98.0%
- ATORVASTATIN 阿伐他汀
- 阿托伐他汀(阿伐他汀)
- (3S,5S)-7-[2-(4-氟苯基)-3-苯基-4-(苯基氨基甲酰基)-5-异丙基吡咯-1-基]-3,5-二羟基庚酸
- 110862-48-1
- 7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid
- Atorvastatin Impurity 153
- (rel)-Atorvastatin
- Atorvastatin isomer
- calcium 7-[4-[anilino(oxo)methyl]-2-(4-fluorophenyl)-3-phenyl-5-propan-2-yl-1-pyrrolyl]-3,5-dihydroxyheptanoate
- Atorvastatin, ?>98.0%
- Atorvastatin(Relative)
- Atorvastatin
- (3S,5S)-7-[2-(4-Fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid
- 1H-PYRROLE-1-HEPTANOIC ACID
- 1H-Pyrrole-1-heptanoic acid, 2-(4-fluorophenyl)-β,δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-, (βR,δR)-rel-