ChemicalBook > CAS DataBase List > Bufexamac
Bufexamac
Bufexamac
- CAS No.2438-72-4
- Chemical Name:Bufexamac
- CBNumber:CB7768637
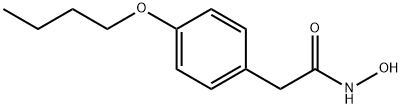
- Molecular Formula:C12H17NO3
- Formula Weight:223.27
- MOL File:2438-72-4.mol
Bufexamac Property
- Melting point 153-155°
- Boiling point 364.56°C (rough estimate)
- Density 1.1223 (rough estimate)
- refractive index 1.5300 (estimate)
- storage temp. Inert atmosphere,Store in freezer, under -20°C
- solubility Practically insoluble in water, soluble in dimethylformamide, slightly soluble in ethyl acetate and in methanol.
- form Solid
- pka 9.24±0.20(Predicted)
- color White to Off-White
- Merck 14,1474
- CAS DataBase Reference 2438-72-4(CAS DataBase Reference)
- FDA UNII 4T3C38J78L
- ATC code M01AB17,M02AA09
- UNSPSC Code 41116107
- NACRES NA.24
Safety
- WGK Germany :2
- RTECS :AK8280000
- HS Code :2928.00.2500
- Toxicity :LD50 orally in mice, rats: >8, >4 g/kg (Lambelin)
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H317
- Precautionary statements P261-P272-P280-P302+P352+P333+P313+P363-P501
Bufexamac Price
More Price(2)
- Brand: Sigma-Aldrich(India)
- Product number: B0760
- Product name : Bufexamac
- Purity: analytical standard
- Packaging: 10G
- Price: ₹10262.1
- Updated: 2022/06/14
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: B4179
- Product name : Bufexamac
- Purity:
- Packaging: 5G
- Price: ₹4600
- Updated: 2022/05/26
- Buy: Buy
Bufexamac Chemical Properties,Usage,Production
- Chemical Properties White Solid
- Originator Parfenac,Lederle,UK,1973
- Uses antiinflammatory, analgesic, antipyretic
- Uses Bufexamac is a non-steroidal topical medication used for treatment of a variety of problems in which the skin is inflamed. These vary widely from insect bites to bums, plant stings and a wide variety of medical conditions induding psoriasis, hemorrhoidal symptoms, eczema and inflammation of the skin following radiotherapy. In this last case, it can be used before radiotherapy to help prevent inflammation of the skin caused by radiation therapy.
- Uses Bufexamac is a specific inhibitor of class IIB histone deacetylases (HDAC6 and HDAC10). Bufexamac is a non-steroidal anti-inflammatory drug used topically as well as rectally. Formulations containing Bufexamac is used by many patients with eczematous disorders as an alternative to topical corticosteroids.
- Definition ChEBI: A hydroxamic acid derived from phenylacetamide in which the benzene moiety is substituted at C-4 by a butoxy group. It has anti-inflammatory, analgesic, and antipyretic properties.
-
Manufacturing Process
(1) 136 g of p-hydroxyacetophenone, 140 g of butyl bromide, 152 g of
potassium carbonate, 17 g of potassium iodide and 275 cc of ethanol are
mixed and then refluxed for 48 hours. The reaction mixture is cooled, diluted
with water, then extracted with ether. The ethereal phase is washed with a 10% sodium hydroxide solution, then with water, followed by drying, ether is
evaporated and the product distilled under reduced pressure. 168 g of pbutyloxyacetophenone
are obtained with yield of 87% (160°-162°C at 11 mm
Hg).
(2) 192 g of p-butyloxyacetophenone, 42 g of sulfur and 130 g of morpholine are mixed and then refluxed for 14 hours. The resulting solution is poured into water and stirred until crystallization of the sulfurated complex. The latter is filtered, washed with water and dried, Production: 270 g (88% yield). (3) 200 g of sodium hydroxide are dissolved in 1,500 cc of ethanol and then 293 g of the thus-obtained sulfurated complex are added. The mixture is refluxed overnight, The mixture is distilled to separate the maximum of the alcohol and then diluted with water. The resulting solution is acidified with hydrochloric acid, and extracted with ether. The ethereal phase is washed with water, followed by extraction with a 10% sodium carbonate solution. The carbonated solution is acidified with 10% hydrochloric acid, and the resulting precipitate of p-n-butyloxyphenylacetic acid is filtered and dried. 100 g of this product are obtained (70% yield).
(4) 208 g of p-n-butyloxyphenylacetic acid, 368 g of ethanol and 18 cc of sulfuric acid are refluxed for 5 hours. The mixture is diluted with water, after which it is extracted with ether. The ethereal phase is successively washed with water, then with carbonate, and again with water, following which it is dried and distilled to remove solvent. The ester is then distilled at a reduced pressure. 200 g of ethyl p-butyloxyphenylecetate are thus obtained with yield of 61% (186°C at 8 mm Hg).
(5) 7 g of hydroxylamine hydrochloride are dissolved in 100 cc of methanol. A solution of 5 g of sodium in 150 cc of methanol is added and the salt precipitate is separated by filtration. 22 g of ethyl p-n-butyloxyphenylacetate are added to the filtrate and the mixture is refluxed for 1 hour. The mixture is cooled and acidified with 20% hydrochloric acid. 14.7 g of p-nbutyloxyphenylacetohydroxamic acid are thus obtained with yield of 71% (melting point: 153°-155°C). - brand name Anderm (Wyeth-Ayerst); Paraderm (Wyeth-Ayerst); Parfenac (Wyeth-Ayerst);Bufemac;Bufexamac-ratiopharm (r) creme;Bufexine ratiopharm(r) f-sable;Calmaderm;Droxan;Droxaryl zalf 50 mg;Duradermal;Flogocid gel n.n;Flogocid sable;Malipuran;Parafenac (r) milch;Parafenac 5% creme;Parafenac basishad;Parafenac sable;Parafenal;Parfenal creme derm;Viafen u est.crema 40 g.
- Therapeutic Function Antiinflammatory, Analgesic, Antipyretic
- World Health Organization (WHO) Bufexamac, an analgesic and anti-inflammatory agent, was introduced in 1974 for the topical treatment of a wide range of dermatoses. The drug is widely marketed and the World Health Organization is not aware of restrictive action having been taken elsewhere.
- Contact allergens Bufexamac is an arylacetic nonsteroidal anti-inflammatory drug. It induces allergic contact dermatitis, eczematous or erythema multiforme like type, and even generalized eruptions like acute generalized exanthematous pustulosis.
Bufexamac Preparation Products And Raw materials
Raw materials
Preparation Products
Global(261)Suppliers
-
Supplier:
Wuhan Fortuna Chemical Co., Ltd
- Tel:+86-027-59207850
- Email:info@fortunachem.com
- Country:China
- ProdList:5971
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
Nanjing Dolon Biotechnology Co.,Ltd.
- Tel:18905173768
- Email:sales@dolonchem.com
- Country:CHINA
- ProdList:2972
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
Xiamen Shindano Biotechnology Co.,ltd
- Tel:0592-6266840<br/>15750707980
- Email:sales@chemsdano.com
- Country:China
- ProdList:291
- Advantage:58
-
Supplier:
SIMAGCHEM CORP
- Tel: +86-13806087780
- Email:sale@simagchem.com
- Country:China
- ProdList:17365
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
ANHUI WITOP BIOTECH CO., LTD
- Tel: +8615255079626
- Email:eric@witopchemical.com
- Country:China
- ProdList:23541
- Advantage:58
2438-72-4, BufexamacRelated Search:
- Acetamide Methylparaben Citric acid Styrene Butadiene Rubber Phenoxyacetyl chloride Acetohydroxamic acid Ethyl acetohydroxamate 2-Hydroxyphenylacetic acid 3-Hydroxybenzoic acid Vanadyl acetylacetonate 4-Hydroxybenzoic acid 4-Hydroxybenzaldehyde 4-Hydroxy-D-(-)-2-phenylglycine N-Acetylsulfanilyl chloride 2,3-Butanedione monoxime 2-Phenylacetamide 2-HYDROXYACETAMIDE Ascoric Acid
- Lipid signaling
- Miscellaneous
- Chemistry
- NORFEMAC
- API
- 抑制剂
- 中间体产品
- 药靶配体
- 配体家族
- 化学试剂
- 氨基酸衍生物
- 医用原料
- 功能性添加剂化工原料
- 原料
- 标准品
- 原料
- 医药化工类
- 无机盐
- 中药对照品
- 医药原料
- 小分子抑制剂
- 神经信号
- 神经系统用药
- 医药中间体
- BI - BZ
- Analytical Chromatography Product Catalog
- Alphabetic
- Analytical Standards
- C12H17NO3
- 丁苯羟酸,10 MM DMSO 溶液
- 丁苯羟酸对照品
- 布非沙美
- 2-(4-丁氧基苯基)-N-羟基乙酰胺
- 丁苯羟酸, 一种COX抑制剂
- 丁苯羟酸 标准品
- 丁苯羟酸 EP标准品
- BUFEXAMAC,CERTIFIED 标准品
- 丁苯羟酸BUFEXAMAC
- 对丁氧苯乙酰氧肟酸
- 4-丁氧基-N-羟基苯乙酰胺
- 丁苯羟酸
- 2438-72-4
- Bufexamac, 10 mM in DMSO
- Bufexamac, ≥ 98.0%
- Bufexamac (Bufexamic acid)
- Bufexamac USP/EP/BP
- Benzeneacetamide, 4-butoxy-N-hydroxy-
- Bufexamac >
- Bufexamac CRS
- tantalum(2+)
- Bufexamac, 98%, a COX inhibitor
- norfemac
- mofenar
- malipuran
- j3
- flogocidnplastigel
- flogicid
- feximac