ChemicalBook > CAS DataBase List > Ciclopirox
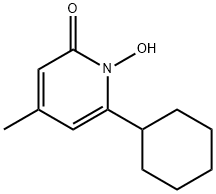
Ciclopirox
Ciclopirox
- CAS No.29342-05-0
- Chemical Name:Ciclopirox
- CBNumber:CB7673863
- Molecular Formula:C12H17NO2
- Formula Weight:207.27
- MOL File:29342-05-0.mol
Ciclopirox Property
- Melting point 1440C
- Boiling point 350.0±25.0 °C(Predicted)
- Density 1.193±0.06 g/cm3(Predicted)
- storage temp. Keep in dark place,Sealed in dry,Room Temperature
- solubility Slightly soluble in water, freely soluble in anhydrous ethanol and in methylene chloride
- pka 6.25±0.58(Predicted)
- form Solid
- color Off-White
- CAS DataBase Reference 29342-05-0(CAS DataBase Reference)
- EWG's Food Scores 1
- FDA UNII 19W019ZDRJ
- ATC code D01AE14,G01AX12
- UNSPSC Code 41116107
- NACRES NA.77
Safety
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302+H312+H332-H319
- Precautionary statements P261-P280-P301+P312-P302+P352+P312-P304+P340+P312-P305+P351+P338
Ciclopirox Price
More Price(3)
- Brand: Sigma-Aldrich(India)
- Product number: SML2011
- Product name : Ciclopirox
- Purity: ≥98% (HPLC)
- Packaging: 50MG
- Price: ₹6679.03
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: SML2011
- Product name : Ciclopirox
- Purity: ≥98% (HPLC)
- Packaging: 100MG
- Price: ₹12080.7
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: PHR1920
- Product name : Ciclopirox
- Purity: Pharmaceutical Secondary Standard; Certified Reference Material
- Packaging: 500MG
- Price: ₹20621.63
- Updated: 2022/06/14
- Buy: Buy
Ciclopirox Chemical Properties,Usage,Production
-
Topical antifungal
Ciclopirox is a novel broad-spectrum topical antifungal agent,it is successfully developed by pharmaceutical companies of the Federal Republic of Germany , the mechanism is by changing the integrity of the fungal cell membrane, causing intracellular material outflow, and blocking intake of protein precursors, resulting in fungal cell death, it has a strong bactericidal effect against dermatophytes, yeasts, molds, etc. And it has strong permeability . At higher concentrations,it can also have a certain extent inhibiting effect on a variety of actinomycetes, Gram-positive and Gram-negative bacteria and mycoplasma, chlamydia, trichomonas, Trichomonas vaginalis and Pseudomonas aeruginosa.
Compared with imidazole antifungal, ciclopirox has strong penetration ability in cuticle where skin fungus survive, so it has a significant inhibition effect on the cuticle deep fungi, such as onychomycosis.
Mainly it is used in clinical for superficial skin fungal infections, such as ringworm, athlete's foot, jock itch, tinea manus and pedis(especially keratosis thickening), tinea versicolor, skin candidiasis, Candida albicans and onychomycosis treatment.
The above information is edited by the chemicalbook of Tian Ye. -
Chemical Properties
Solid, m.p. 144 ℃.
Ciclopirox Olamine: [41621-49-2]. White crystalline powder, odorless, bitter taste. Soluble in methanol, ethanol or chloroform, slightly soluble in dimethylformamide or water, slightly soluble in ether. Melting point 124-128 ℃. Acute toxicity LD50 in mice, rats (mg/kg): 2898,3290 orally. - Uses Pyridine ketones broad-spectrum antifungal agents, have inhibiting effects on a variety of skin fungi . They can prevent the substances necessary for Candida albicans cells survival such as amino acids, potassium ions, phosphate,going through the cell membrane ,which can cause by certain important matrix or intracellular ions failure,and then inhibit or kill fungal cells. There are a wide range of anti-fungal effects, they can have a killing effect on the skin filamentous bacteria, yeast fungi , they have inhibition effects on a variety of Gram positive and negative bacteria, chlamydia, trichomoniasis, Proteus, E. coli, Pseudomonas and Staphylococcus bacteria. They are used to treat fungal skin disease, tinea versicolor, vulvovaginal Candida disease, skin and fingers (toe) candidiasis ,they have significant effects , and lower side effects.
-
production method
4-methyl-3-penten-2-one by oxidation of sodium hypochlorite (56% yield), is esterified to produce methyl-2-butenoate (the I), 67% yield, b.p. 135~139 ℃.
Cyclohexane carboxylic acid and thionyl chloride react to obtain cyclohexane carboxylic acid chloride. In the role of aluminum chloride,it reacts with methyl-2-butenoate (I) in a methylene chloride solvent, and the reaction is stirred for 4h, after post-treatment, vacuum collect 140-145 ℃ (0.4kPa) the distillate, 3-methyl-5-oxo-5-cyclohexyl-3-pentenoate (ciclopirox, II) is generated in 75% yield. And then it is stirred at room temperature for 20h together with hydroxylamine hydrochloride, sodium acetate, methanol and water , then it is added 50% sodium hydroxide , then it is stirred for 1h. After cooling with benzene extraction, the aqueous phase is acidified to Ph = 6. The precipitated crystals are recrystallized from ethanol, to give ciclopirox, m.p. 140~142 ℃, yield 48.3%. Finally,it is salified with the hydroxyl amine in methylene chloride to give almost quantitative ciclopirox, melting point 97~99 ℃. - Chemical Properties White or Light-Yellow Powder
- Originator Fungirox Esmalte, UCI-Farma
- Uses Broad spectrum antimycotic agent with some antibacterial activity.
- Uses Ciclopirox (Penlac) is a synthetic antifungal agent. Ciclopirox (Penlac) is used for topical dermatologic treatment of superficial mycoses. Ciclopirox (Penlac) is most useful against Tinea versicolor. [1] In a study conducted to further elucidate ciclopir
- Indications Ciclopirox penetrates rapidly into and through the skin and nail keratin. After dermal application ofa1% cream formulation, about 1.3 % of the dose is absorbed. Serum levels range around 10 μg/L after application of a dose of 37 mg of ciclopirox. After vaginal administration, 15 – 20 % of the dose is absorbed. Ciclopirox penetrates well into the skin and nail matrix. Absorbed ciclopirox is mainly eliminated by the kidneys; 1.1 – 1.7 % of the topical dose is eliminated in the urine within four days. Only a fraction thereof is unaltered ciclopirox. Approximately 96% of the absorbed drug is bound to human serum proteins.
- Definition ChEBI: A cyclic hydroxamic acid that is 1-hydroxypyridin-2(1H)-one in which the hydrogens at positions 4 and 6 are substituted by methyl and cyclohexyl groups, repectively. A broad spectrum antigfungal agent, it also exhibits antibacterial acti ity against many Gram-positive and Gram-negative bacteria, and has anti-inflammatory properties. It is used a a topical treatment of fungal skin and nail infections.
-
Manufacturing Process
A mixture of 5-oxo-3-methyl-5-cyclohexylpentene-2 acid 1-methyl ester and
5-oxo-3-methyl-5-cyclohexylpentene-3 acid 1-methyl ester was obtained by
condensation of hexahydrobenzoyl chloride with β,β-dimethylacrylic acid
methyl ester. 11.2 g of this mixture and a solution of 4.6 g of sodium acetate
and 4 g of hydroxylamine hydrochloride were shaken for 20 hours at 25°C
with a mixture of 8 ml of water and 15 ml methanol. Subsequently, a solution
of 4 g of sodium hydroxide in 8 ml of water was then added, while cooling,
shaken for 1 hour at room temperature. The mixture was extracted by means
of benzene and the aqueous phase was acidified to reach a pH of 6. 3.5 g of
1-hydroxy-4-methyl-6-cyclohexyl-2-pyridone were obtained; melting point
144°C.
For preparation of cyclopirox from 1-hydroxy-4-methyl-6-cyclohexyl-2- pyridone was added 2-aminoethanol (1:1).Merck Index, Monograph number: 2325, Twelfth edition, 1996, Editor: S. Budavari; Merck and Co., Inc. Greene L.A.; US Patent No. 5,846,984; Dec. 8, 1998; Assigned to The Trustees of Columbia University in the City of New York (New York, NY) Lohaus G. et al.; US Patent No. 3,883,545; May 13, 1975; Assigned to Hoechst Aktiengesellschaft, Frankfurt am Main, Germany - brand name Loprox(Medicis); Penlac (Sanofi Aventis).
- Therapeutic Function Antifungal
- Antimicrobial activity Ciclopirox has a broad in vitro antifungal activity spectrum, including dermatophytes, yeasts, and moulds. The most important fungi within the activity spectrum of ciclopirox. Ciclopirox is primarily fungistatic, but if the contact time is long enough and the concentration exceeds 20 μg/mL, it exerts a fungicidal effect on yeasts and dermatophytes. In vitro, the effect is limited to proliferating fungi and is greatly dependent on the nutrient medium.
- Biological Activity Ciclopirox is a chemical compound belonging to the class of hydroxypyridones. It is found active against dermatophytes, yeasts, molds, some bacterias and also azole-resistant Candida species. Unlike other antifungal agents th at act for ergosterol inhibition, ciclopirox has a different action. It targets metal-dependent enzymes, which degrades fungal cell peroxides. This unique action of ciclopirox provides less chance for resistance in pathogenic fungi. This compound is used in topical formulations specifically for nails and skin.', 'Ciclopirox is an antifungal and cell-permeable iron-chelating agent. It has been found to inhibit multiple enzymes and signaling pathways including prolyl hydroxylase 2 (PHD2), eukaryotic translation initiation factor 5A (eIF5A), Wnt/β-catenin, hypoxia-inducible factor-1α (HIF-1 α)/vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor 3 (VEGFR-3), mammalian target of rapamycin (mTOR), and cyclin dependent kinases (CDKs). Ciclopirox has been shown to have anti-viral and anticancer activity in addition to its antifungal activity. Ciclopirox has also been shown to counteract glucotoxicity in diabetic mice in a p21-dependent manner.
- Mechanism of action Ciclopirox has a unique mechanism of action through chelation of polyvalent cations, such as Fe3+, which causes inhibition of a number of metal-dependent enzymes within the fungal cell.
- Mechanism of action Ciclopirox is a substituted pyridione derivative. Experiments show that the mechanism of action involves a disruption of transport mechanisms into the cell plasma, especially the uptake of leucine. Further, the uptake of other amino acids such as phenylalanine and lysine, as well as the uptake of potassium and phosphate ions is impaired. This all probably involves a change in membrane permeability, as reported for some other antimycotics, although ciclopirox does not show lytic activity. Consecutively, the substance significantly reduces fungal growth rates.
- Clinical Use Ciclopirox is a hydroxylated pyridinone that is employed for superficial dermatophytic infections, principally onychomycosis.
- Side effects Topically applied, ciclopirox is well tolerated. The incidence of local irritations such as itching and burning sensations range between 1 and 4 % and are often due to the galenic formulation.
- storage Store at +4°C
Ciclopirox Preparation Products And Raw materials
Raw materials
Preparation Products
Global(358)Suppliers
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12811
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531151365
- Email:mina@chuanghaibio.com
- Country:China
- ProdList:18137
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
Xiamen AmoyChem Co., Ltd
- Tel:+86-86-5926051114<br/>+8615060885618
- Email:sales@amoychem.com
- Country:China
- ProdList:6383
- Advantage:58
-
Supplier:
BOC Sciences
- Tel:+1-631-485-4226
- Email:inquiry@bocsci.com
- Country:United States
- ProdList:19552
- Advantage:58
-
Supplier:
Chongqing Chemdad Co., Ltd
- Tel:+86-023-6139-8061<br/>+86-86-13650506873
- Email:sales@chemdad.com
- Country:China
- ProdList:39894
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
Zhengzhou Alfa Chemical Co.,Ltd
- Tel: +8618530059196
- Email:sale04@alfachem.cn
- Country:China
- ProdList:12162
- Advantage:58
-
Supplier:
SIMAGCHEM CORP
- Tel: +86-13806087780
- Email:sale@simagchem.com
- Country:China
- ProdList:17365
- Advantage:58
Related articles
29342-05-0, CiclopiroxRelated Search:
- Paraquat dichloride Methanol 2,4,6-Collidine Azamethiphos METHYLCYCLOHEXANOL 1-Hydroperoxycyclohexyl-1-hydroxycyclohexyl peroxide Methyl acrylate CHLOROPHOSPHONAZO III 2-Methyl-2-propenoic acid cyclohexyl ester 3-Hydroxypyridine Methylparaben Kresoxim-methyl 2-Cyclohexen-1-one 4-Methylpyridine METHYLCYCLOHEXANONE Cyclohexanone Acetonitrile Basic Violet 1
- Heterocycles
- Batrafen, Loprox, Mycoster, Stieprox, HOE 296b
- API
- Pharmaceuticals
- Intermediates & Fine Chemicals
- 科研原料
- 抑制剂
- 药靶配体
- 合成有机化合物配体
- 化工原材料
- 化学试剂
- 医药原料及中间体
- 精细化工原料
- 日用化学品
- 有机化工原料
- 精细化工品
- 医药原料药
- 合成抗菌药
- 科研试剂
- 其他原料
- 医用原料
- 医药原料药-科研原料
- 医药化工类
- 农业原料
- 药物
- 抗真菌感染药物
- 抗病源性微生物药
- 原料药
- 小分子抑制剂
- 原料
- 医药原料
- CICLOPIROX 环吡酮胺 |CAS 29342-05-0
- 环吡酮,10 MM DMSO 溶液
- 甲醇中环比酮胺
- 环吡酮胺检测标准品
- 甲醇中环匹罗司标准溶液
- PERFEMIKER]环吡酮胺,99%
- 异帕米星杂质5
- 13C6]-环吡酮
- L-萝卜硫素/R-萝卜硫素
- 环吡酮 USP标准品
- 环吡酮 EP标准品
- 环匹罗司(CAS号:29342-05-0)
- 环吡酮-D11
- 环吡酮胺 50MG
- 环匹罗司(标准品)
- 6-环己基-1-羟基-4-甲基-2(IH)-吡啶酮
- 环己吡酮氨乙醇
- 环吡酮胺
- 6-环己基-1-羟基-4-甲基-2(1H)-吡啶酮
- 环匹罗司胺
- 环己吡酮羟乙胺盐
- 环己吡酮羟乙胺
- 环吡司
- 环匹罗司
- 环吡酮
- 29342-05-0
- Ciclopirox, 10 mM in DMSO