ChemicalBook > CAS DataBase List > Cefdinir
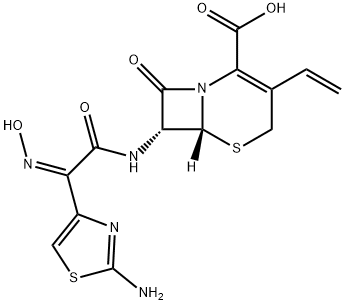
Cefdinir
Cefdinir
- CAS No.91832-40-5
- Chemical Name:Cefdinir
- CBNumber:CB7483101
- Molecular Formula:C14H13N5O5S2
- Formula Weight:395.41
- MOL File:91832-40-5.mol
Cefdinir Property
- Melting point >180°C dec.
- Density 1.89±0.1 g/cm3(Predicted)
- storage temp. Keep in dark place,Sealed in dry,2-8°C
- solubility dilute HCl: slightly soluble
- pka 9.70(at 25℃)
- form solid
- color Pale Yellow to Light Yellow
- Water Solubility Soluble in water
- λmax 295nm(DMSO)(lit.)
- Merck 14,1920
- BCS Class 4
- InChIKey RTXOFQZKPXMALH-GHXIOONMSA-N
- SMILES N12[C@@]([H])([C@H](NC(/C(/C3=CSC(N)=N3)=N\O)=O)C1=O)SCC(C=C)=C2C(O)=O
- CAS DataBase Reference 91832-40-5(CAS DataBase Reference)
- FDA UNII CI0FAO63WC
- ATC code J01DD15
Safety
-
Symbol(GHS)

- Signal wordDanger
- Hazard statements H317-H334
- Precautionary statements P261-P272-P280-P284-P302+P352+P333+P313+P363-P304+P340+P342+P311-P501
Cefdinir Price
More Price(3)
- Brand: Sigma-Aldrich(India)
- Product number: C7118
- Product name : Cefdinir
- Purity: ≥97.0% (HPLC)
- Packaging: 1G
- Price: ₹10413.65
- Updated: 2022/06/14
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: C3111
- Product name : Cefdinir
- Purity:
- Packaging: 1G
- Price: ₹7600
- Updated: 2022/05/26
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: C3111
- Product name : Cefdinir
- Purity:
- Packaging: 5G
- Price: ₹14800
- Updated: 2022/05/26
- Buy: Buy
Cefdinir Chemical Properties,Usage,Production
- Brand Name(s) in US Omnicef
- Description Cefdinir is a cephalosporin antibiotic. It is active against numerous Gram-positive and Gram-negative bacteria, including β-lactamase-producing E. coli, K. oxytoca, K. pneumoniae, and P. aeruginosa clinical isolates (MICs = 0.25-16 μg/ml). Cefdinir is protective against sepsis induced by strains of S. aureus or H. influenzae in mice with 50% protective dose (PD50) values of 2.7-35 and 3.1-5.8 mg/kg, respectively. Formulations containing cefdinir have been used in the treatment of Gram-positive and Gram-negative infections.
- Description Cefdinir is an orally active, beta-lactamase stable cephalosporin with a broad spectrum of activity. Compared to other oral cephalosporins, cefdinir is more potent against Gram-positive bacteria, especially Staphylococci. Its activity against Gram-negative bacteria such as E.coli,K. pneumoniae and P.mirabilis is similar to cefixime, but superior to cefaclor and cephalexin.
- Chemical Properties Pale Yellow Solid
- Originator Fujisawa (Japan)
- Uses A broad spectrum antibiotic targeting both Gram-positive and Gram-negative pathogens
- Uses antihypertensive, ACE inhibitor
- Uses A Cephalosporin antibiotic structurally similar to Cefixime
- Definition ChEBI: A cephalosporin compound having 7beta-2-(2-amino-thiazol-4-yl)-2-[(Z)-hydroxyimino]-acetylamino- and 3-vinyl side groups.
-
Manufacturing Process
By interaction of 7-amino-8-oxo-3-vinyl-5-thia-1-azabicyclo(4.2.0)oct-2-ene-
2-carboxylic acid 4-methoxyphenyl ester with 4-bromoacetyl bromide was
prepared 7-(4-bromo-3-oxo-butyrylamino)-8-oxo-3-vinyl-5-thia-1-azabicyclo
(4.2.0)oct-2-ene-2-carboxylic acid 4-methoxyphenyl ester. The active
methylene group in that product was then nitrosated with sodium nitrite. The
initial product spontaneously tautomerizes to afford 7-(4-bromo-2-
hydroxyimino-3-oxo-butyrylamino)-8-oxo-3-vinyl-5-thia-1-azabicyclo(4.2.0)
oct-2-ene-2-carboxylic acid 4-methoxyphenyl ester. By the reaction of that
compound with thiourea and then with trifluoroacetic acid was obtained
(6R,7R)-7-(2-(2-amino-4-thiazolyl)glyoxylamido)-8-oxo-3-vinyl-5-thia-1-
azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid sodium nitrite, (Z)-oxime
(Cefdinir sodium nitrile).
In practice it is usually used as free acid.
Synthesis of 7β-[2-(2-aminothiazol-4-yl)-2-(Z)-(trytiloxyimino)acetamido]-3- vinyl-3-cephem-4-carboxylic acid x p-toluenesulfonic acid x 2 N,N-dimethylacetamide (the precursor of Cefdinir) was described in Patent US 6,093,814. - brand name Cefzon
- Therapeutic Function Antibiotic
-
Antimicrobial activity
An oral cephalosporin similar in structure to cefixime, but
with a slightly modified side chain at the 7-amino position.
Activity is similar to that of cefixime, but it is more active,
especially against staphylococci. It is not hydrolyzed
by staphylococcal or the common plasmid-mediated
enterobacterial β-lactamases. An enhancing effect on phagocytosis
has been demonstrated in vitro.
Oral absorption is about 35%. A 200 mg oral dose achieves a plasma concentration of 1 mg/L after c. 3 h. Absorption is reduced after a fatty meal. Concentrations equal to or higher than corresponding plasma levels were present in blister fluid 6–12 h after administration of an oral dose. The plasma halflife is 1.5 h. Protein binding is 60–70%. A total of 12–20% of the dose was excreted in the urine within 12 h, the renal elimination declining with increasing dose. The elimination half-life and peak plasma concentration are increased in renal failure. About 60% of the drug is removed by hemodialysis.
Side effects and uses are those common to oral cephalosporins. - Safety Profile Moderately toxic by ingestion andintravenous routes. Low toxicity by intraperitoneal andsubcutaneous routes. Experimental reproductive effects.When heated to decomposition it emits toxic vapors ofNOx and SOx.
Cefdinir Preparation Products And Raw materials
Raw materials
Preparation Products
Global(496)Suppliers
-
Supplier:
Shenzhen Excellent Biomedical Technology Co.,Ltd.
- Tel: +undefined18318671572
- Email:sale@ex-biotech.com
- Country:China
- ProdList:1092
- Advantage:58
-
Supplier:
Hebei Jingbo New Material Technology Co., Ltd
- Tel: +8619931165850
- Email:hbjbtech@163.com
- Country:China
- ProdList:1000
- Advantage:58
-
Supplier:
Hebei Saisier Technology Co., LTD
- Tel:+86-18400010335<br/>+86-18034520335
- Email:admin@hbsaisier.cn
- Country:China
- ProdList:1015
- Advantage:58
-
Supplier:
Shaanxi TNJONE Pharmaceutical Co., Ltd
- Tel:+86-17396673057
- Email:linda@tnjone.com
- Country:China
- ProdList:1143
- Advantage:58
-
Supplier:
Hebei Longbang Technology Co., LTD
- Tel:+86-18633929156<br/>+86-18633929156
- Email:admin@hblongbang.com
- Country:China
- ProdList:972
- Advantage:58
-
Supplier:
HebeiShuoshengImportandExportco.,Ltd
- Tel:+86-18532138899<br/>+86-18532138899
- Email:L18532138899@163.com
- Country:China
- ProdList:939
- Advantage:58
-
Supplier:
Shaanxi Xianhe Biotech Co., Ltd
- Tel: +8617709210191
- Email:Jerry@xhobio.com
- Country:China
- ProdList:884
- Advantage:58
-
Supplier:
Capot Chemical Co.,Ltd.
- Tel:+86-(0)57185586718<br/>+86-13336195806
- Email:sales@capot.com
- Country:China
- ProdList:29730
- Advantage:60
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
Related articles
91832-40-5, CefdinirRelated Search:
- Glycine 4-(4-CHLORO-BENZYLOXY)-PHENYLAMINE N-Vinyl-2-pyrrolidone VINYL PROPIONATE ALTRENOGEST Triethoxyvinylsilane CHLOROPHOSPHONAZO III Betaine Silicone rubber,methyl-vinyl Vinyl ester resin Vinyl resin Cefixime Ceftiolene Cefditoren Cefditoren pivoxil (Z)-2-(5-AMINO-1,2,4-THIADIAZOL-3-YL)-2-METHOXYIMINO ACETIC ACID (Benzothiazol-2-yl)-(Z)-2-trityloxyimino-2-(2-aminothiazole-4-yl)-thioacetate YTTERBIUM(III) IONOPHORE I
- Sulfur & Selenium Compounds
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Miscellaneous Biochemicals
- ACEON
- Chiral Reagents
- cephalosporins
- 信号转导通路激酶抑制剂
- 头孢类试剂
- 杂质对照品
- 抑制剂
- 药靶配体
- 化工中间体
- 化工中间体-化工原料
- 精细化工原料
- 工业化
- 抗生素类
- 化药杂质-头孢地尼
- 有机化工原料
- 化工原料
- 医药、农药及染料中间体
- 原料
- 医用原料
- 对照品-杂质对照品
- 原料中间体-原料药
- 中间体
- 医药化工类
- 医药 抗菌药
- 原料药
- 化学试剂
- 试剂
- 原料药及其中间体
- 药物
- 抗生素
- β-内酰胺类抗生素
- 医药中间体
- 小分子抑制剂
- 医药原料药
- 医药原料
- C14H13N5O5S2
- 1832-40-5
- 头孢地尼,10 MM DMSO 溶液
- PERFEMIKER]头孢地尼,98%
- (6R,7R)-7-[[(2-氨基-4-噻唑基)-(肟基)乙酰基]氨基]-3-乙烯基-8-氧代-5-硫杂-1-氮杂双环[4.2.0]辛-2-烯-2-羧酸
- (6R,7R)-7-((Z)-2-(2-氨基噻唑-4-基)-2-(肟基)乙酰胺基)-8-氧代-3-烯基-5-硫杂-1-氮杂双环[4.2.0]辛-2-烯-2-羧酸
- (6R,7R)-7-((Z)-2-(2-氨基噻唑-4-基)-2-(羟基亚氨基)乙酰胺基)-8-氧代-3-乙烯基-5-硫杂-1-氮杂双环[4.2.0]辛-2-烯-2-羧酸
- 头孢地尼-13C-15N2
- 头孢地尼 USP标准品
- CEFDINIR头孢地尼
- 头孢地尼母核
- [6R-[6Α,7Β(Z)]]-7-[[(2-氨基-4-噻唑基)(羟基亚氨)乙酰基]氨基]-3-乙烯基-8-氧代-5-硫杂-1-氮杂二环[4.2.0]-2-辛烯-2-羧酸
- 头孢地尼/[6R-[6Α,7Β(Z)]]-7-[[(2-氨基-4-噻唑基)(羟基亚氨)乙酰基]氨基]-3-乙烯基-8-氧代-5-硫杂-1-氮杂二环[4.2.0]-2-辛烯-2-羧酸
- 头孢地尼 1G
- 头孢地尼
- (6R,7R)-7-[(Z)-2-(2-氨基-4-噻唑基)-2-羟基亚胺基乙酰氨基]-8-氧代-3-乙烯基-5-硫杂-1-氮杂双环[4.2.0]辛-2-烯-2-羧酸
- 头孢地尼侧链(ATAABTE)
- 91832-40-5
- Cefdinir, 10 mM in DMSO