ChemicalBook > CAS DataBase List > Tazobactam acid
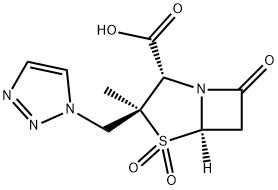
Tazobactam acid
Tazobactam acid
- CAS No.89786-04-9
- Chemical Name:Tazobactam acid
- CBNumber:CB7473784
- Molecular Formula:C10H12N4O5S
- Formula Weight:300.29
- MOL File:89786-04-9.mol
Tazobactam acid Property
- Melting point 115-145℃
- Boiling point 77℃
- Density 1.92±0.1 g/cm3(Predicted)
- RTECS XI0191400
- Flash point >110°(230°F)
- storage temp. Sealed in dry,Store in freezer, under -20°C
- solubility DMSO (Slightly, Heated), Methanol (Slightly, Heated, Sonicated)
- form Solid
- pka 2.33±0.40(Predicted)
- color White to Off-White
- Water Solubility Soluble in water
- Stability Light Sensitive
- InChI InChI=1S/C10H12N4O5S/c1-10(5-13-3-2-11-12-13)8(9(16)17)14-6(15)4-7(14)20(10,18)19/h2-3,7-8H,4-5H2,1H3,(H,16,17)/t7-,8+,10+/m1/s1
- InChIKey LPQZKKCYTLCDGQ-WEDXCCLWSA-N
- SMILES N12[C@@]([H])(CC1=O)S(=O)(=O)[C@@](C)(CN1C=CN=N1)[C@@H]2C(O)=O
- CAS DataBase Reference 89786-04-9(CAS DataBase Reference)
- FDA UNII SE10G96M8W
- ATC code J01CG02
Safety
-
Symbol(GHS)

- Signal wordDanger
- Hazard statements H315-H319-H335
- Precautionary statements P261-P280a-P304+P340-P305+P351+P338-P405-P501a
Tazobactam acid Price
More Price(3)
- Brand: Sigma-Aldrich(India)
- Product number: PHR1686
- Product name : Tazobactam
- Purity: Pharmaceutical Secondary Standard; Certified Reference Material
- Packaging: 1G
- Price: ₹9493.53
- Updated: 2022/06/14
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: T3732
- Product name : Tazobactam
- Purity:
- Packaging: 1G
- Price: ₹4300
- Updated: 2022/05/26
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: T3732
- Product name : Tazobactam
- Purity:
- Packaging: 5G
- Price: ₹10000
- Updated: 2022/05/26
- Buy: Buy
Tazobactam acid Chemical Properties,Usage,Production
- Description Tazobactam often is coadministered with piperacillin because of tazobactam's ability to inhibit β-lactamases. T azobactam, like other β-lactamase inhibitors, has little or no antibacterial activity. This effect is analogous to that of clavulanic acid and sulbactam.
- Chemical Properties White or off-white powder
- Originator CL-307579,China Pharm Chemical Co.,,China
- Uses Tazobactam is a β-Lactamase inhibitor, used with β-lactam antibiotics to enhance their effect.
- Uses b-lactamase inhibitor
- Definition ChEBI: A member of the class of penicillanic acids that is sulbactam in which one of the exocyclic methyl hydrogens is replaced by a 1,2,3-triazol-1-yl group; used (in the form of its sodium salt) in combination with ceftolozane sulfate for treatment of complicat d intra-abdominal infections and complicated urinary tract infections.
-
Manufacturing Process
A known β-lactam type antibiotic (for example, benzhydryl 2-β-chloromethyl-
2-α-methylpenam-3-α-carboxylate) was used for synthesis of new penicillinic
derivatives.
A solution of 5.00 g of sodium azide in 53 ml of water was added to a solution of benzhydryl 2-β-chloromethyl-2-α-methylpenam-3-α-carboxylate (5.13 g) in dimethylformamide (155 ml). The mixture was stirred at room temperature for 4 h. The resulting reaction mixture was poured into cooled water and the mixture was extracted with ethyl acetate. The ethyl acetate layer was washed with water, dried over magnesium sulfate and concentrated to provide 4.87 g of the benzhydryl 2-β-azidomethyl-2-α-methylpenam-3-α-carboxylate as oil in 93% yield.
To a solution of benzhydryl 2-β-azidomethyl-2-α-methylpenam-3-α- carboxylate (7.03 g) in a mixture of acetic acid (240 ml) and water (40 ml) was added potassium permanganate (6.02 g) over a period of more than 1 h. The mixture was stirred at room temperature for 2.5 h. The resulting reaction mixture was diluted with ice water. The precipitate was collected by filtration, and washed with water. The resulting product was dissolved in ethyl acetate and the solution was washed with an aqueous solution of sodium hydrogen carbonate and dried over magnesium sulfate. Concentration gave 5.48 g of the benzhydryl 2-β-azidomethyl-2-α-methylpenam-3-α-carboxylate-1,1-dioxide in 72% yield.
A 200 mg quantity of benzhydryl 2-β-azidomethyl-2-α-methylpenam-3-α- carboxylate-1,1-dioxide was reacted with 10 ml of vinyl acetate in a sealed reactor at 100° to 110°C for 30 h. The reaction mixture was concentrated at reduced pressure. The residue was crystallized with cooled chloroform. The white crystals of benzhydryl 2-α-methyl-2-β-(1,2,3-triazol-1-yl)methylpenam- 3-α-carboxylate-1,1-dioxide have a melting point 206°-208°C, dec.
Hydrogenation was conducted in 10 ml of ethyl acetate and 10 ml of water at room temperature for 30 min by using 45 mg of benzhydryl 2-α-methyl-2-β- (1,2,3-triazol-1-yl)methylpenam-3-α-carboxylate-1,1-dioxide, 15 mg of 10% palladium charcoal and 16 mg of sodium hydrogen carbonate. The aqueous layer was separated from the reaction mixture and washed once with ethyl acetate. The aqueous solution was then purified with an MCl gel, CHP-20P (product of Mitsubishi Kasei Co., Ltd., Japan). After freeze-drying, there was obtained an amorphous product of sodium 2-α-methyl-2-β-(1,2,3-triazol-1- yl)methylpenam-3-α-carboxylate-1,1-dioxide with a melting point 170°C, dec. - Therapeutic Function Antibiotic
- Antimicrobial activity Tazobactam exhibits little useful antimicrobial activity, although weak activity against Acinetobacter spp. and Borrelia burgdorferi has been reported.
- Mechanism of action Tazobactam functions as an irreversible inhibitor by covalently binding to β-lactamases, and prevents the enzyme hydrolysis of ceftolozane, thereby enhancing its activity against resistant ESBL-producing pathogens, along with expanding coverage to include anaerobes (e.g., Bacteroides spp.)
-
Clinical Use
Tazobactam is a penicillanic acid sulfone that is similar instructure to sulbactam. It is a more potent β-lactamaseinhibitor than sulbactam and has a slightly broader spectrumof activity than clavulanic acid. It has very weak antibacterialactivity. Tazobactam is available in fixed-dose, injectablecombinations with piperacillin, a broad-spectrum penicillinconsisting of an 8:1 ratio of piperacillin sodium to tazobactamsodium by weight and marketed under the trade name Zosyn.The pharmacokinetics of the two drugs are very similar. Bothhave short half-lives (t1/2 ~1 hour), are minimally proteinbound, experience very little metabolism, and are excreted inactive forms in the urine in high concentrations.
Approved indications for the piperacillin–tazobactamcombination include the treatment of appendicitis, postpartumendometritis, and pelvic inflammatory disease caused byβ-lactamase–producing E. coli and Bacteroides spp., skin andskin structure infections caused by β-lactamase–producingS. aureus, and pneumonia caused by β-lactamase–producingstrains of H. influenzae. - Veterinary Drugs and Treatments Although veterinary experience is limited with piperacillin or piperacillin/ tazobactam, these drugs have expanded coverage against many bacteria and may be suitable for empiric use until culture and susceptibility data are available, or for surgical prophylaxis when gram-negative or mixed aerobic/anaerobic infections are concerns.
-
Drug interactions
Potentially hazardous interactions with other drugs
Reduced excretion of methotrexate - monitor methotrexate levels during concomitant treatment.
Enhanced action of vecuronium and similar neuromuscular blocking agents. -
Metabolism
Piperacillin is metabolised to a minor microbiologically
active desethyl metabolite. Tazobactam is metabolised
to a single metabolite that has been found to be
microbiologically inactive.
Piperacillin and tazobactam are eliminated via the kidney by glomerular filtration and tubular secretion. Piperacillin, tazobactam, and desethyl piperacillin are also secreted into the bile.
Tazobactam acid Preparation Products And Raw materials
Raw materials
Preparation Products
Global(365)Suppliers
-
Supplier:
Hpets Biotech Company (Chongqing) Co.,ltd
- Tel: +8613340353813
- Email:info@hanpets.com
- Country:China
- ProdList:26
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12811
- Advantage:58
-
Supplier:
Hangzhou Hyper Chemicals Limited
- Tel:+86-0086-57187702781<br/>+8613675893055
- Email:info@hyper-chem.com
- Country:China
- ProdList:295
- Advantage:58
-
Supplier:
Shaanxi TNJONE Pharmaceutical Co., Ltd
- Tel:+86-17396673057
- Email:linda@tnjone.com
- Country:China
- ProdList:1143
- Advantage:58
-
Supplier:
Moxin Chemicals
- Tel: +8617320513646
- Email:Anna@molcoo.com
- Country:China
- ProdList:9534
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Nanjing ChemLin Chemical Industry Co., Ltd.
- Tel:025-83697070
- Email:product@chemlin.com.cn
- Country:CHINA
- ProdList:3009
- Advantage:60
-
Supplier:
Shanghai Yingrui Biopharma Co., Ltd.
- Tel:+86-21-33585366 - 03@
- Email:sales03@shyrchem.com
- Country:CHINA
- ProdList:738
- Advantage:60
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418679<br/>+8618949832763
- Email:info@tnjchem.com
- Country:China
- ProdList:2986
- Advantage:55
Related articles
89786-04-9, Tazobactam acidRelated Search:
- Citric acid 1,2,4-Triazole TAZOBACTAM SODIUM,TAZOBACTAM SODIUM SALT 5-Methyl-1H-benzotriazole Ethyl 2-(Chlorosulfonyl)acetate Acetonitrile Tolyltriazole Paraquat dichloride Methylparaben Basic Violet 1 1H-Benzotriazole Glycine Folic acid Methanol Kresoxim-methyl Methyl acetate Methyl acrylate 1,2,3-1H-Triazole
- pharmaceutical
- Peptide Synthesis/Antibiotics
- Amino Acid Derivatives
- TAZOCIN
- 高纯试剂
- 杂质对照品
- 科研原料
- 抑制剂
- 生化试剂
- 化工原料
- 医药原料药API
- API医药原料
- 临床检测标准物质
- 科研精品试剂
- 化药杂质-他唑巴坦
- 抗生素类
- 化学试剂
- 医药、农药及染料中间体
- 原料
- 药物杂质及中间体
- 医药原料
- 原料药API
- 中间体
- 医药化工类
- 对照品-杂质对照品
- 原料药
- 小分子抑制剂
- 药物
- 抗生素
- β-内酰胺类抗生素
- 医药原料药
- C10H12N4O5S
- 他唑巴坦,10 MM DMSO 溶液
- TAZOBACTAMUM|||TAZOBACTAM ACID|||他唑巴坦酸|||YTR-830H|||CL-298741|||他唑巴坦
- (2S,3S,5R)-3-[(1H-1,2,3-三唑-1-基)甲基]-3-甲基-7-氧代-4-硫杂-1-氮杂双环[3.2.0]庚烷-2-甲酸-4,4-二氧化物
- TAROBACTAM ACID 他唑巴坦酸
- [2S-(2Α,3Β,5Α)]-3-甲基-7-氧代-3-(1H-1,2,3-三唑-1-基甲基)-4-硫杂-1-氮杂双环[3.2.0]庚烷-2-羧酸-4,4-二氧化物
- 他唑巴坦酸/他唑巴坦/
- (2S,3S,5R)-3-((1H-1,2,3-三唑-1-基)甲基)-3-甲基-7-氧代-4-硫杂-1-氮杂双环[3.2.0]庚烷-2-羧酸 4,4-二氧化物
- 他唑巴坦酸 USP标准品
- 泰
- 他唑巴坦酸(三唑巴坦)
- [2S-(2a,3b,5a)]-3-甲基-7-氧代-3-(1H-1,2,3-三氮唑-1-基甲基)-4-硫代-1-氮杂双环[3,2,0]庚烷-2-羧酸 4,4-二氧化物
- 他唑巴坦(三唑巴坦、他佐巴坦)
- 他唑巴坦(标准品)
- 他唑巴坦,他唑巴坦酸
- 2α-甲基-2β-(1,2,3-三氮唑-1-基)甲基青霉烷砜-3α-羧酸
- 三唑巴坦
- 他佐巴坦
- 三唑烷砜
- 三唑克坦
- 泰唑巴坦酸
- 泰唑巴坦
- 他唑巴坦
- 他唑巴坦酸
- 89786-04-9
- TAZOBACTAM (NON-STERILE API)
- TAZOBACTAM USP